Sunday Poster Session
Category: IBD
P1120 - Comparison of Serum IL-22 and Tissue Molecular Changes Between Guselkumab Subcutaneous and Intravenous Induction in Moderately to Severely Active Crohn’s Disease: Post Hoc Analysis of the GRAVITI and GALAXI Studies
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
Has Audio

Patrick Branigan, BS
Johnson & Johnson
Spring House, PA
Presenting Author(s)
Klebea Sohn, PhD1, Dylan Richards, PhD1, Ruchi Patel, MS1, Amy Hart, PhD2, Mobolaji Olurinde, MD, PhD3, Nat A. Terry, MD, PhD4, Bradford McRae, PhD1, Walter Reinisch, MD, PhD5, Flavio Steinwurz, MD, MACG6, Remo Panaccione, MD7, Geert R. D’Haens, MD, PhD8, Patrick Branigan, BS4
1Janssen Research & Development, LLC, Spring House, PA, USA, Spring House, PA; 2Janssen Research & Development, LLC,, Spring House, PA; 3Janssen Research & Development, LLC, Spring House, PA; 4Johnson & Johnson, Spring House, PA; 5Medical University of Vienna, Department of Internal Medicine III, Division of Gastroenterology and Hepatology, Spitalgasse, Wien, Austria; 6Hospital Israelita Albert Einstein, São Paulo, Sao Paulo, Brazil; 7Inflammatory Bowel Disease Unit, Division of Gastroenterology and Hepatology, Department of Medicine, University of Calgary, Calgary, AB, Canada, Calgary, AB, Canada; 8Department of Gastroenterology, Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands
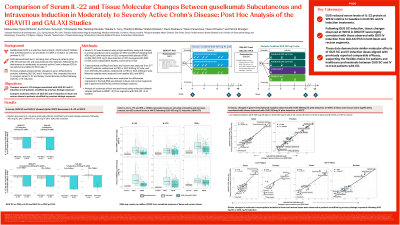
Introduction: Guselkumab (GUS) is a selective dual-acting IL-23p19 subunit inhibitor that blocks IL-23 and binds to CD64, a receptor on cells that produce IL-23. GUS demonstrated short- and long-term efficacy with intravenous (IV) and subcutaneous (SC) induction dosing followed by SC maintenance treatment in patients (pts) with moderately to severely active Crohn’s disease (CD) in the GALAXI and GRAVITI Ph3 trials. Here, we present a comparison of the tissue molecular effects of GUS SC and GUS IV induction treatment in CD.
Methods: Serum IL-22 was measured in a subset of participants and changes in protein abundance were assessed at WK0 and WK12 following GUS 400 mg SC q4w induction (n=202) compared with placebo (PBO) (n=86) (GRAVITI) or, GUS 200 mg IV q4w induction (n=50) compared with PBO (n=50) (GALAXI), and healthy control sera (n=30). Transcriptional profiling of ileum and rectum segments was performed using samples from 277 GRAVITI patients randomized to PBO or GUS 400 mg SC, and from a subset of GALAXI patients (n=259) randomized to PBO or GUS 200mg IV at baseline (BL) and WK12. Gene co-expression modules were evaluated in ileum and rectum segments, and in patients stratified by prior history of inadequate response to biologics.
Results: GUS SC and IV induction showed similar WK12 decreases in serum IL-22 levels and is consistent with reductions in IFNγ, IL-17A, CRP and fecal calprotectin levels (p< 0.05) previously reported. In tissue, changes in gene modules at WK12 observed with GUS 400mg SC q4w induction were significantly correlated with those observed with GUS 200mg IV q4w induction (R=0.97, p< 0.05 in ileum and R=0.93, p< 0.05 in rectum). Similar effects were observed in patients stratified by previous biologic exposure. GUS SC induction reduced key cellular and inflammatory transcriptional modules at WK12 including plasma cell, inflammatory epithelial, neutrophil, and IL-23/Th17 biology (FDR< 0.05), consistent with molecular analyses of GUS IV induction. Similar changes were observed for tissue IFNγ, IL-17A, and IL-22 gene expression with levels approaching those of the non-IBD controls at WK12.
Discussion: GUS SC and IV induction regimens demonstrate similar molecular effects. Transcriptional changes observed with GUS SC induction in GRAVITI were highly correlated with those observed with GUS IV induction from GALAXI Ph3 in ileum and rectum segments. These data are consistent with previously reported results demonstrating comparable efficacy between GUS SC and IV induction regimen.
Disclosures:
Klebea Sohn, PhD1, Dylan Richards, PhD1, Ruchi Patel, MS1, Amy Hart, PhD2, Mobolaji Olurinde, MD, PhD3, Nat A. Terry, MD, PhD4, Bradford McRae, PhD1, Walter Reinisch, MD, PhD5, Flavio Steinwurz, MD, MACG6, Remo Panaccione, MD7, Geert R. D’Haens, MD, PhD8, Patrick Branigan, BS4. P1120 - Comparison of Serum IL-22 and Tissue Molecular Changes Between Guselkumab Subcutaneous and Intravenous Induction in Moderately to Severely Active Crohn’s Disease: Post Hoc Analysis of the GRAVITI and GALAXI Studies, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Janssen Research & Development, LLC, Spring House, PA, USA, Spring House, PA; 2Janssen Research & Development, LLC,, Spring House, PA; 3Janssen Research & Development, LLC, Spring House, PA; 4Johnson & Johnson, Spring House, PA; 5Medical University of Vienna, Department of Internal Medicine III, Division of Gastroenterology and Hepatology, Spitalgasse, Wien, Austria; 6Hospital Israelita Albert Einstein, São Paulo, Sao Paulo, Brazil; 7Inflammatory Bowel Disease Unit, Division of Gastroenterology and Hepatology, Department of Medicine, University of Calgary, Calgary, AB, Canada, Calgary, AB, Canada; 8Department of Gastroenterology, Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands
Introduction: Guselkumab (GUS) is a selective dual-acting IL-23p19 subunit inhibitor that blocks IL-23 and binds to CD64, a receptor on cells that produce IL-23. GUS demonstrated short- and long-term efficacy with intravenous (IV) and subcutaneous (SC) induction dosing followed by SC maintenance treatment in patients (pts) with moderately to severely active Crohn’s disease (CD) in the GALAXI and GRAVITI Ph3 trials. Here, we present a comparison of the tissue molecular effects of GUS SC and GUS IV induction treatment in CD.
Methods: Serum IL-22 was measured in a subset of participants and changes in protein abundance were assessed at WK0 and WK12 following GUS 400 mg SC q4w induction (n=202) compared with placebo (PBO) (n=86) (GRAVITI) or, GUS 200 mg IV q4w induction (n=50) compared with PBO (n=50) (GALAXI), and healthy control sera (n=30). Transcriptional profiling of ileum and rectum segments was performed using samples from 277 GRAVITI patients randomized to PBO or GUS 400 mg SC, and from a subset of GALAXI patients (n=259) randomized to PBO or GUS 200mg IV at baseline (BL) and WK12. Gene co-expression modules were evaluated in ileum and rectum segments, and in patients stratified by prior history of inadequate response to biologics.
Results: GUS SC and IV induction showed similar WK12 decreases in serum IL-22 levels and is consistent with reductions in IFNγ, IL-17A, CRP and fecal calprotectin levels (p< 0.05) previously reported. In tissue, changes in gene modules at WK12 observed with GUS 400mg SC q4w induction were significantly correlated with those observed with GUS 200mg IV q4w induction (R=0.97, p< 0.05 in ileum and R=0.93, p< 0.05 in rectum). Similar effects were observed in patients stratified by previous biologic exposure. GUS SC induction reduced key cellular and inflammatory transcriptional modules at WK12 including plasma cell, inflammatory epithelial, neutrophil, and IL-23/Th17 biology (FDR< 0.05), consistent with molecular analyses of GUS IV induction. Similar changes were observed for tissue IFNγ, IL-17A, and IL-22 gene expression with levels approaching those of the non-IBD controls at WK12.
Discussion: GUS SC and IV induction regimens demonstrate similar molecular effects. Transcriptional changes observed with GUS SC induction in GRAVITI were highly correlated with those observed with GUS IV induction from GALAXI Ph3 in ileum and rectum segments. These data are consistent with previously reported results demonstrating comparable efficacy between GUS SC and IV induction regimen.
Disclosures:
Klebea Sohn: Johnson and Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Dylan Richards: Johnson and Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Ruchi Patel: Johnson and Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Amy Hart: Johnson and Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Mobolaji Olurinde: Johnson and Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Nat Terry: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Bradford McRae: Johnson and Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Walter Reinisch: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support. Actelion – Advisory Committee/Board Member, Consultant. Alpha Wasserman – Advisory Committee/Board Member, Consultant. AstraZeneca – Advisory Committee/Board Member, Consultant. Cellerix – Advisory Committee/Board Member, Consultant. Cosmo Pharmaceuticals – Advisory Committee/Board Member, Consultant. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant. Genentech – Advisory Committee/Board Member, Consultant. Grunenthal – Advisory Committee/Board Member, Consultant. Johnson & Johnson – Advisory Committee/Board Member, Consultant. Merck – Advisory Committee/Board Member, Consultant. Millennium – Advisory Committee/Board Member, Consultant. Novo Nordisk – Advisory Committee/Board Member, Consultant. Nycomed – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Pharmacosmos – Advisory Committee/Board Member, Consultant. Salix Pharmaceuticals – Advisory Committee/Board Member, Consultant. Schering-Plough – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant. UCB Pharma – Advisory Committee/Board Member, Consultant. Vifor Pharma – Advisory Committee/Board Member, Consultant.
Flavio Steinwurz: Abbvie – Consultant, Speaker, researcher. Amgen – Consultant, Speaker, researcher. Celltrion – Consultant, Speaker, researcher. Ferring – Consultant, Speaker, researcher. Johnson & Johnson – Consultant, Speaker, researcher. Pfizer – Consultant, Speaker, researcher. Sandoz – Consultant, Speaker, researcher. Takeda – Consultant, Speaker, researcher.
Remo Panaccione: Abbott – Consultant. AbbVie – Advisory Committee/Board Member, Consultant, Speaker's fees. Abivax – Consultant. Alimentiv – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant, Speaker's fees. AnaptysBio – Consultant. Arena Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. AstraZeneca – Advisory Committee/Board Member, Consultant. Biogen – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker's fees. Celgene – Advisory Committee/Board Member, Consultant, Speaker's fees. Celltrion – Consultant. Cosmos Pharmaceuticals – Consultant. Eisai – Consultant. Elan Pharmaceuticals – Consultant. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Speaker's fees. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. Fresenius Kabi – Advisory Committee/Board Member, Consultant, Speaker's fees. Galapagos – Consultant. Genentech (Roche) – Advisory Committee/Board Member, Consultant. Gilead Sciences – Advisory Committee/Board Member, Consultant, Speaker's fees. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. JAMP Pharma – Advisory Committee/Board Member, Consultant. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Speaker's fees. Merck & Co., Inc. – Advisory Committee/Board Member, Consultant, Speaker's fees. Mirador – Consultant. Mylan – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Odyssey – Consultant. Oppilan Pharma – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant, Speaker's fees. Pandion Therapeutics – Advisory Committee/Board Member, Consultant. Pendopharm G.I. Solutions – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker's fees. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Biosciences – Consultant. Protagonist Therapeutics Inc – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant, Speaker's fees. Sandoz – Advisory Committee/Board Member, Consultant, Speaker's fees. Sanofi – Consultant. Satisfai Health – Consultant. Shire Pharma – Advisory Committee/Board Member, Consultant, Speaker's fees. Spyre Therapeutics – Consultant. Sublimity Therapeutics – Advisory Committee/Board Member, Consultant. Takeda Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. Teva – Consultant. Theravance Biopharma – Consultant. Tillots – Consultant. Trellus Health – Consultant. UCB – Consultant. Union Biopharma – Consultant. Ventyx Biosciences – Advisory Committee/Board Member, Consultant. Viatris – Consultant.
Geert D’Haens: Abbvie – Advisor or Review Panel Member, Speakers Bureau. Abivax – Advisor or Review Panel Member. Agomab – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. AMT – Consultant. Anaptys Bio – Advisor or Review Panel Member. AstraZeneca – Consultant. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member. Eli Lilly – Advisor or Review Panel Member. Exeliom – Consultant. Galapagos – Advisor or Review Panel Member. Glaxo Smith Kline – Advisor or Review Panel Member. Gossamerbio – Consultant. Immunic – Consultant. Index Pharmaceuticals – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member. Kaleido – Consultant. Merck – Advisor or Review Panel Member. Origo – Consultant. Pfizer – Consultant, Speakers Bureau. Polpharm – Advisor or Review Panel Member. Procise Diagnostics – Consultant. Progenity – Consultant. Prometheus Biosciences – Advisor or Review Panel Member. Protagonist Therapeutics – Consultant. Sanofi – Advisor or Review Panel Member. Seres Health – Advisory Committee/Board Member. Takeda – Advisor or Review Panel Member. Tillotts – Advisor or Review Panel Member.
Patrick Branigan: Johnson & Johnson – Employee, Stock-publicly held company(excluding mutual/index funds).
Klebea Sohn, PhD1, Dylan Richards, PhD1, Ruchi Patel, MS1, Amy Hart, PhD2, Mobolaji Olurinde, MD, PhD3, Nat A. Terry, MD, PhD4, Bradford McRae, PhD1, Walter Reinisch, MD, PhD5, Flavio Steinwurz, MD, MACG6, Remo Panaccione, MD7, Geert R. D’Haens, MD, PhD8, Patrick Branigan, BS4. P1120 - Comparison of Serum IL-22 and Tissue Molecular Changes Between Guselkumab Subcutaneous and Intravenous Induction in Moderately to Severely Active Crohn’s Disease: Post Hoc Analysis of the GRAVITI and GALAXI Studies, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
