Sunday Poster Session
Category: Infections and Microbiome
P1286 - Evaluation of Fecal Microbiota, Live-jslm by Standard of Care Antibiotic Washout Period for the Prevention of Recurrent Clostridioides difficile Infection
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Has Audio
- TR
Timothy Ritter, MD
GI Alliance
Southlake, TX
Presenting Author(s)
Timothy E. Ritter, MD1, Jonathan A. Rosenberg, MD2, Richard L. Hengel, MD3, Sujatha Krishnan, MD4, Kathy A.. Baker, PhD, APRN, ACNS-BC5, Lucinda J.. Van Anglen, PharmD6, Kelly E.. Hanna, PharmD6, Mielad Moosapanah, PharmD7, Sanghyuk Seo, PharmD7, Kevin W.. Garey, PharmD, MS8
1GI Alliance, Southlake, TX; 2GI Alliance of Illinois, Highland Park, IL; 3Atlanta ID Group, Atlanta, GA; 4DFW Infectious Diseases, PLLC, Frisco, TX; 5Harris College of Nursing and Health Sciences, Texas Christian University, Fort Worth, TX; 6Healix Infusion Therapy, LLC, Sugar Land, TX; 7Ferring Pharmaceuticals, Inc., Parsippany, NJ; 8University of Houston College of Pharmacy, Houston, TX
Introduction: Fecal microbiota, live-jslm (RBL) was approved in November 2022 as the first microbiome-based product for the prevention of recurrent Clostridioides difficile infection (rCDI). RBL is indicated to be rectally administered after 24-72 hours of washout from the standard of care (SoC) antibiotic for rCDI. Various factors contribute to the timely administration of RBL in real-world practice. The study aims to report the outcomes of RBL-treated patients, comparing those receiving RBL within 24-72 hours to those receiving RBL after this timeframe.
Methods: This retrospective, multicenter study included patients ≥18 years with rCDI who received RBL in US physician offices from 2/2023 to 4/2025 with 8-week follow-up data. Centralized electronic health records were reviewed for patient demographics, comorbidities, rCDI history, risk factors, RBL therapy characteristics, and SoC antibiotic washout period. Recurrence was defined as ≥3 occurrences of diarrhea within 24 hours and was assessed at 8 weeks post-RBL. Patients were evaluated based on the antibiotic washout period: ≤3 days following completion of SoC (Group 1) and >3 days up to 42 days following completion of SoC (Group 2). Pearson’s Chi-Square test was used to test for statistical significance.
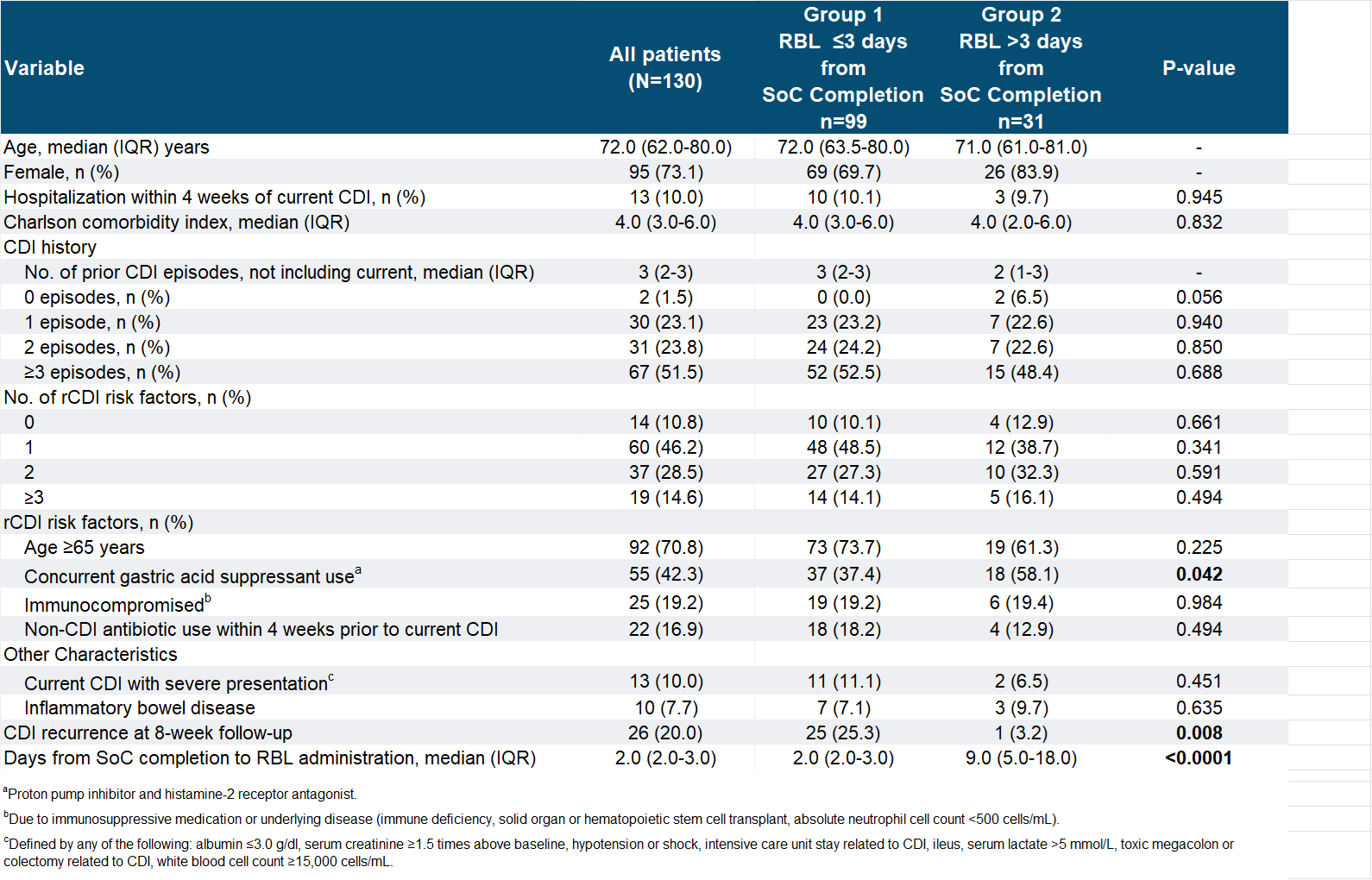
Results: Of the 130 patients who received RBL, 99 patients were in Group 1 and 31 patients were in Group 2 (Table 1). The median days of washout period were 2 days in Group 1 and 9 days in Group 2 (p< 0.0001). The two groups had a similar median age (Group 1: 72; Group 2: 71), same median Charlson Comorbidity Index of 4.0, with female being the majority in both. Most patients in both groups had at least one risk factor for rCDI, but higher numbers of patients aged ≥65 (74% vs. 61%) and non-CDI antibiotic use (18% vs. 13%) were observed in Group 1, while a higher number of patients using gastric acid suppressants was observed in Group 2 (37% vs. 58%). More patients in Group 1 had a severe presentation of CDI than Group 2 (11% vs. 7%). The proportion of patients with inflammatory bowel disease was similar across the groups. Recurrence was seen in 25.3% in Group 1 vs. 3.2% in Group 2, where the difference was statistically significant (p=0.008).
Discussion: These data suggest that RBL continues to be effective in preventing recurrences despite being administered beyond 72 hours after SoC antibiotic washout. Further analysis with a larger cohort is needed to confirm these early results.
Disclosures:
Timothy E. Ritter, MD1, Jonathan A. Rosenberg, MD2, Richard L. Hengel, MD3, Sujatha Krishnan, MD4, Kathy A.. Baker, PhD, APRN, ACNS-BC5, Lucinda J.. Van Anglen, PharmD6, Kelly E.. Hanna, PharmD6, Mielad Moosapanah, PharmD7, Sanghyuk Seo, PharmD7, Kevin W.. Garey, PharmD, MS8. P1286 - Evaluation of Fecal Microbiota, Live-jslm by Standard of Care Antibiotic Washout Period for the Prevention of Recurrent <i>Clostridioides difficile</i> Infection, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1GI Alliance, Southlake, TX; 2GI Alliance of Illinois, Highland Park, IL; 3Atlanta ID Group, Atlanta, GA; 4DFW Infectious Diseases, PLLC, Frisco, TX; 5Harris College of Nursing and Health Sciences, Texas Christian University, Fort Worth, TX; 6Healix Infusion Therapy, LLC, Sugar Land, TX; 7Ferring Pharmaceuticals, Inc., Parsippany, NJ; 8University of Houston College of Pharmacy, Houston, TX
Introduction: Fecal microbiota, live-jslm (RBL) was approved in November 2022 as the first microbiome-based product for the prevention of recurrent Clostridioides difficile infection (rCDI). RBL is indicated to be rectally administered after 24-72 hours of washout from the standard of care (SoC) antibiotic for rCDI. Various factors contribute to the timely administration of RBL in real-world practice. The study aims to report the outcomes of RBL-treated patients, comparing those receiving RBL within 24-72 hours to those receiving RBL after this timeframe.
Methods: This retrospective, multicenter study included patients ≥18 years with rCDI who received RBL in US physician offices from 2/2023 to 4/2025 with 8-week follow-up data. Centralized electronic health records were reviewed for patient demographics, comorbidities, rCDI history, risk factors, RBL therapy characteristics, and SoC antibiotic washout period. Recurrence was defined as ≥3 occurrences of diarrhea within 24 hours and was assessed at 8 weeks post-RBL. Patients were evaluated based on the antibiotic washout period: ≤3 days following completion of SoC (Group 1) and >3 days up to 42 days following completion of SoC (Group 2). Pearson’s Chi-Square test was used to test for statistical significance.
Results: Of the 130 patients who received RBL, 99 patients were in Group 1 and 31 patients were in Group 2 (Table 1). The median days of washout period were 2 days in Group 1 and 9 days in Group 2 (p< 0.0001). The two groups had a similar median age (Group 1: 72; Group 2: 71), same median Charlson Comorbidity Index of 4.0, with female being the majority in both. Most patients in both groups had at least one risk factor for rCDI, but higher numbers of patients aged ≥65 (74% vs. 61%) and non-CDI antibiotic use (18% vs. 13%) were observed in Group 1, while a higher number of patients using gastric acid suppressants was observed in Group 2 (37% vs. 58%). More patients in Group 1 had a severe presentation of CDI than Group 2 (11% vs. 7%). The proportion of patients with inflammatory bowel disease was similar across the groups. Recurrence was seen in 25.3% in Group 1 vs. 3.2% in Group 2, where the difference was statistically significant (p=0.008).
Discussion: These data suggest that RBL continues to be effective in preventing recurrences despite being administered beyond 72 hours after SoC antibiotic washout. Further analysis with a larger cohort is needed to confirm these early results.

Figure: Table 1: Patient Characteristics and Treatment Variables
Disclosures:
Timothy E. Ritter: Abbvie – Advisory Committee/Board Member, Speakers Bureau. Boehringer Ingelheim – Advisory Committee/Board Member. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Eli Lilly – Advisory Committee/Board Member, Speakers Bureau. Ferring Pharmaceuticals – Advisory Committee/Board Member. Genentech/Roche – Advisory Committee/Board Member. Gilead – Advisor or Review Panel Member. Intercept – Advisory Committee/Board Member. Iterative Scopes – Advisory Committee/Board Member, Stock-privately held company. Janssen – Advisory Committee/Board Member, Speakers Bureau. Merck & Co. – Advisor or Review Panel Member. Pfizer – Advisory Committee/Board Member, Speakers Bureau. Sanofi – Advisor or Review Panel Member. Spyre Therapeutics – Advisor or Review Panel Member. Takeda Pharmaceuticals – Advisor or Review Panel Member.
Jonathan A. Rosenberg: Aimmune – Advisor or Review Panel Member. Ferring Pharmaceuticals – Advisor or Review Panel Member.
Richard L. Hengel: Gilead – Grant/Research Support.
Sujatha Krishnan indicated no relevant financial relationships.
Kathy Baker indicated no relevant financial relationships.
Lucinda Van Anglen: Cumberland Pharmaceuticals – Grant/Research Support. Ferring Pharmaceuticals – Grant/Research Support. Melinta Therapeutics – Grant/Research Support. Novartis Pharmaceuticals – Grant/Research Support. Takeda Pharmaceuticals – Grant/Research Support.
Kelly Hanna indicated no relevant financial relationships.
Mielad Moosapanah: Ferring Pharmaceuticals – Employee.
Sanghyuk Seo: Ferring Pharmaceuticals – Employee.
Kevin Garey: Acurx – Grant/Research Support. Merck & Co. – Grant/Research Support. Paratek Pharmaceuticals – Grant/Research Support.
Timothy E. Ritter, MD1, Jonathan A. Rosenberg, MD2, Richard L. Hengel, MD3, Sujatha Krishnan, MD4, Kathy A.. Baker, PhD, APRN, ACNS-BC5, Lucinda J.. Van Anglen, PharmD6, Kelly E.. Hanna, PharmD6, Mielad Moosapanah, PharmD7, Sanghyuk Seo, PharmD7, Kevin W.. Garey, PharmD, MS8. P1286 - Evaluation of Fecal Microbiota, Live-jslm by Standard of Care Antibiotic Washout Period for the Prevention of Recurrent <i>Clostridioides difficile</i> Infection, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
