Sunday Poster Session
Category: Infections and Microbiome
P1287 - Risk of Clostridioides difficile Infection Following Rifaximin Treatment for Small Intestinal Bacterial Overgrowth
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
Has Audio

Abdelrahman Yousef, MD
University of New Mexico Hospital
Albuquerque, NM
Presenting Author(s)
Abdelrahman Yousef, MD1, Niven Wang, DO1, Mahmoud Yousef, MD2, Khaled Elfert, MBChB, MRCP3, Ahmed Telbany, MD4, Daniel Peverini, MD1, Shannon Henry, DO1, Eliseo Castillo, PhD4, Aleksandr Birg, MD4
1University of New Mexico Hospital, Albuquerque, NM; 2University of Texas MD Anderson Cancer Center, Houston, TX; 3West Virginia University School of Medicine, Morgantown, WV; 4University of New Mexico, Albuquerque, NM
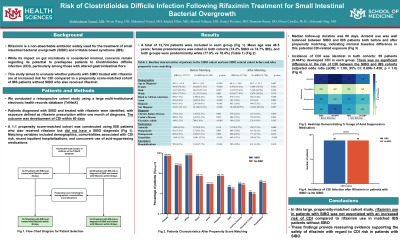
Introduction: Rifaximin is a non-absorbable antibiotic widely used for the treatment of small intestinal bacterial overgrowth (SIBO) and irritable bowel syndrome (IBS). While its impact on gut microbiota is considered minimal, concerns remain regarding its potential to predispose patients to Clostridioides difficile infection (CDI), particularly among those with altered gastrointestinal flora. This study aimed to evaluate whether patients with SIBO treated with rifaximin are at increased risk for CDI compared to a propensity score-matched cohort of IBS patients without SIBO who also received rifaximin.
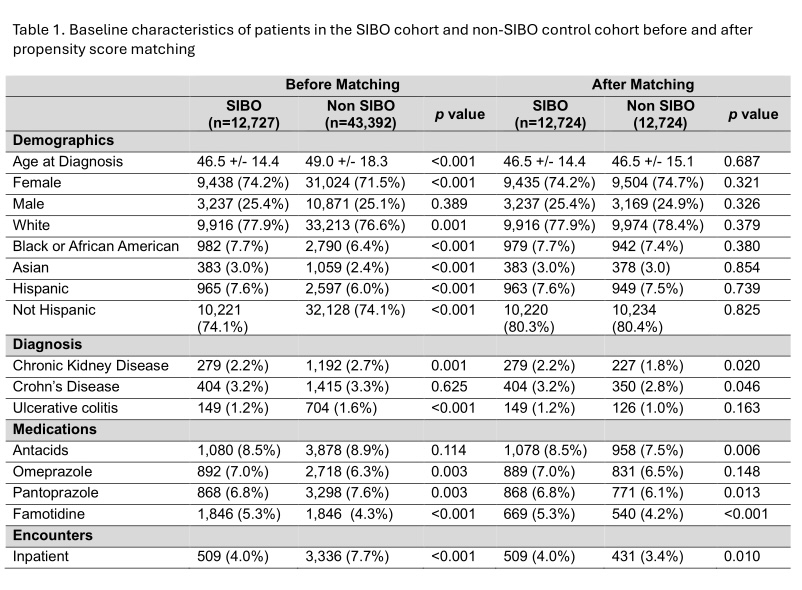
Methods: We conducted a retrospective cohort study using a large multi-institutional electronic health records database (TriNetX). Patients diagnosed with SIBO and treated with rifaximin were identified, with exposure defined as rifaximin prescription within one month of diagnosis. The outcome was development of CDI within 60 days. A 1:1 propensity score-matched cohort was constructed using IBS patients who also received rifaximin but did not have a SIBO diagnosis (Table 1). Matching variables included demographics (age, sex, race), comorbidities associated with CDI risk (Crohn’s disease, ulcerative colitis, chronic kidney disease), recent inpatient hospitalizations, and concurrent use of acid-suppressing medications (omeprazole, pantoprazole, famotidine, and other antacids).
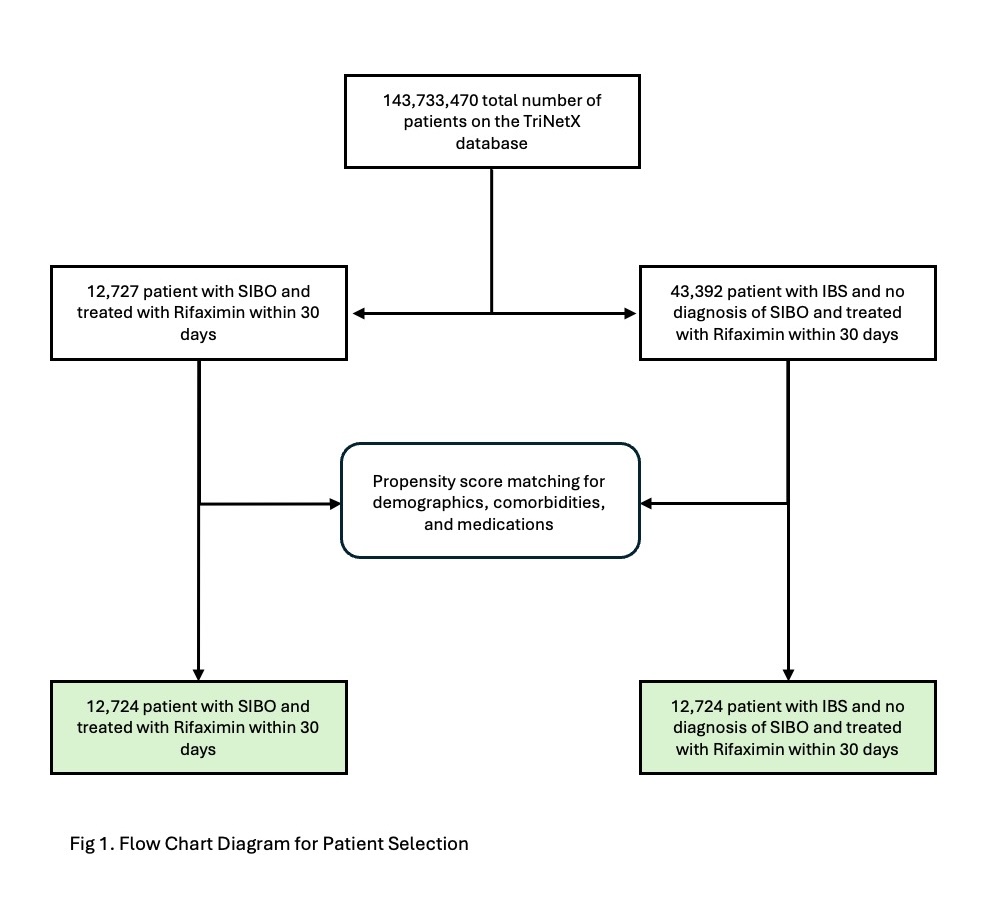
Results: A total of 12,724 patients were included in each group (Figure 1). Mean age was 46.5 years; female predominance was noted in both cohorts (74.2% SIBO vs 74.7% IBS), and both groups were predominantly white (77.9% vs 78.4%) (Table 1). Median follow-up duration was 60 days. Incidence of CDI was identical in both cohorts: 59 patients (0.464%) developed CDI in each group. There was no significant difference in the risk of CDI between the SIBO and IBS cohorts (adjusted odds ratio [aOR] = 1.00, 95% CI: 0.696–1.436; p = 1.0).
Discussion: In this large, propensity-matched cohort study, rifaximin use in patients with SIBO was not associated with an increased risk of CDI compared to rifaximin use in matched IBS patients without SIBO. These findings provide reassuring evidence supporting the safety of rifaximin with regard to CDI risk in patients with SIBO.
Disclosures:
Abdelrahman Yousef, MD1, Niven Wang, DO1, Mahmoud Yousef, MD2, Khaled Elfert, MBChB, MRCP3, Ahmed Telbany, MD4, Daniel Peverini, MD1, Shannon Henry, DO1, Eliseo Castillo, PhD4, Aleksandr Birg, MD4. P1287 - Risk of <i>Clostridioides difficile</i> Infection Following Rifaximin Treatment for Small Intestinal Bacterial Overgrowth, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of New Mexico Hospital, Albuquerque, NM; 2University of Texas MD Anderson Cancer Center, Houston, TX; 3West Virginia University School of Medicine, Morgantown, WV; 4University of New Mexico, Albuquerque, NM
Introduction: Rifaximin is a non-absorbable antibiotic widely used for the treatment of small intestinal bacterial overgrowth (SIBO) and irritable bowel syndrome (IBS). While its impact on gut microbiota is considered minimal, concerns remain regarding its potential to predispose patients to Clostridioides difficile infection (CDI), particularly among those with altered gastrointestinal flora. This study aimed to evaluate whether patients with SIBO treated with rifaximin are at increased risk for CDI compared to a propensity score-matched cohort of IBS patients without SIBO who also received rifaximin.
Methods: We conducted a retrospective cohort study using a large multi-institutional electronic health records database (TriNetX). Patients diagnosed with SIBO and treated with rifaximin were identified, with exposure defined as rifaximin prescription within one month of diagnosis. The outcome was development of CDI within 60 days. A 1:1 propensity score-matched cohort was constructed using IBS patients who also received rifaximin but did not have a SIBO diagnosis (Table 1). Matching variables included demographics (age, sex, race), comorbidities associated with CDI risk (Crohn’s disease, ulcerative colitis, chronic kidney disease), recent inpatient hospitalizations, and concurrent use of acid-suppressing medications (omeprazole, pantoprazole, famotidine, and other antacids).
Results: A total of 12,724 patients were included in each group (Figure 1). Mean age was 46.5 years; female predominance was noted in both cohorts (74.2% SIBO vs 74.7% IBS), and both groups were predominantly white (77.9% vs 78.4%) (Table 1). Median follow-up duration was 60 days. Incidence of CDI was identical in both cohorts: 59 patients (0.464%) developed CDI in each group. There was no significant difference in the risk of CDI between the SIBO and IBS cohorts (adjusted odds ratio [aOR] = 1.00, 95% CI: 0.696–1.436; p = 1.0).
Discussion: In this large, propensity-matched cohort study, rifaximin use in patients with SIBO was not associated with an increased risk of CDI compared to rifaximin use in matched IBS patients without SIBO. These findings provide reassuring evidence supporting the safety of rifaximin with regard to CDI risk in patients with SIBO.

Figure: Figure 1. Flow Chart for Patient Selection

Figure: Table 1. Baseline characteristics of patients in the SIBO cohort and non-SIBO control cohort before and after propensity score matching
Disclosures:
Abdelrahman Yousef indicated no relevant financial relationships.
Niven Wang indicated no relevant financial relationships.
Mahmoud Yousef indicated no relevant financial relationships.
Khaled Elfert indicated no relevant financial relationships.
Ahmed Telbany indicated no relevant financial relationships.
Daniel Peverini indicated no relevant financial relationships.
Shannon Henry indicated no relevant financial relationships.
Eliseo Castillo indicated no relevant financial relationships.
Aleksandr Birg indicated no relevant financial relationships.
Abdelrahman Yousef, MD1, Niven Wang, DO1, Mahmoud Yousef, MD2, Khaled Elfert, MBChB, MRCP3, Ahmed Telbany, MD4, Daniel Peverini, MD1, Shannon Henry, DO1, Eliseo Castillo, PhD4, Aleksandr Birg, MD4. P1287 - Risk of <i>Clostridioides difficile</i> Infection Following Rifaximin Treatment for Small Intestinal Bacterial Overgrowth, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
