Monday Poster Session
Category: Colon
P2450 - Reduced Immunosuppression Requirements With GLP-1 Receptor Agonists Use in Patients Experiencing Immune-Mediated Diarrhea and Colitis
- CN
Cristina Natha, MD
McGovern Medical School at UTHealth
Houston, TX
Presenting Author(s)
1McGovern Medical School at UTHealth, Houston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX; 3MD Anderson Cancer Center, Houston, TX; 4University of Texas Medical Branch, Galveston, TX; 5University of Texas Health Sciences Center in Houston, Houston, TX; 6University of Texas at Houston, Houston, TX; 7Baylor College of Medicine / MD Anderson Cancer Center, Houston, TX; 8Baylor College of Medicine, Houston, TX; 9McGovern Medical School at UTHealth Houston, Houston, TX; 10University of Texas Health, McGovern Medical School, Houston, TX
Introduction:
Metformin, a common therapy for type 2 diabetes mellitus (T2DM), has been associated with worse outcomes in patients who develop immune-mediated diarrhea and colitis (IMDC) during immune checkpoint inhibitor (ICI) therapy, prompting interest in alternative agents. Glucagon-like peptide-1 receptor agonists (GLP-1RA), also used in T2DM, promote insulin secretion, delay gastric emptying, and promote satiety. Notably, GLP-1 receptors are expressed on immune cells, where activation promotes regulatory T cell responses and immune exhaustion gene expression. These immunomodulatory properties extend beyond glycemic control, with benefits in cardiovascular and renal disease. This study evaluated whether GLP-1RA use impacts IMDC severity or outcomes in ICI-treated patients.
Methods:
This retrospective single-center study included ICI-treated patients from 2015-2025. GLP-1RA users were compared with non-users and a historical metformin cohort. Chi-square and Fisher’s exact tests were used (SPSS v24).
Results:
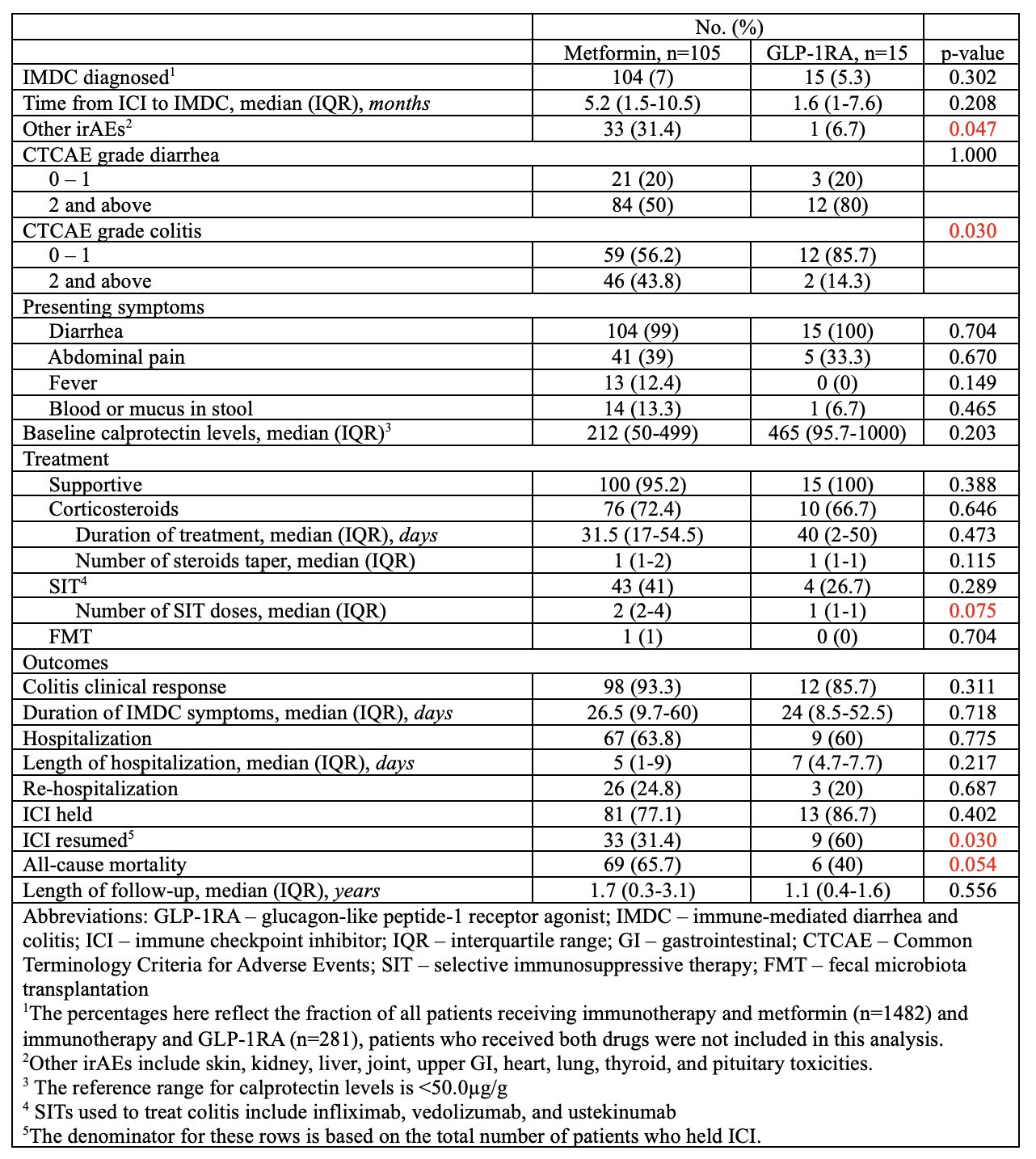
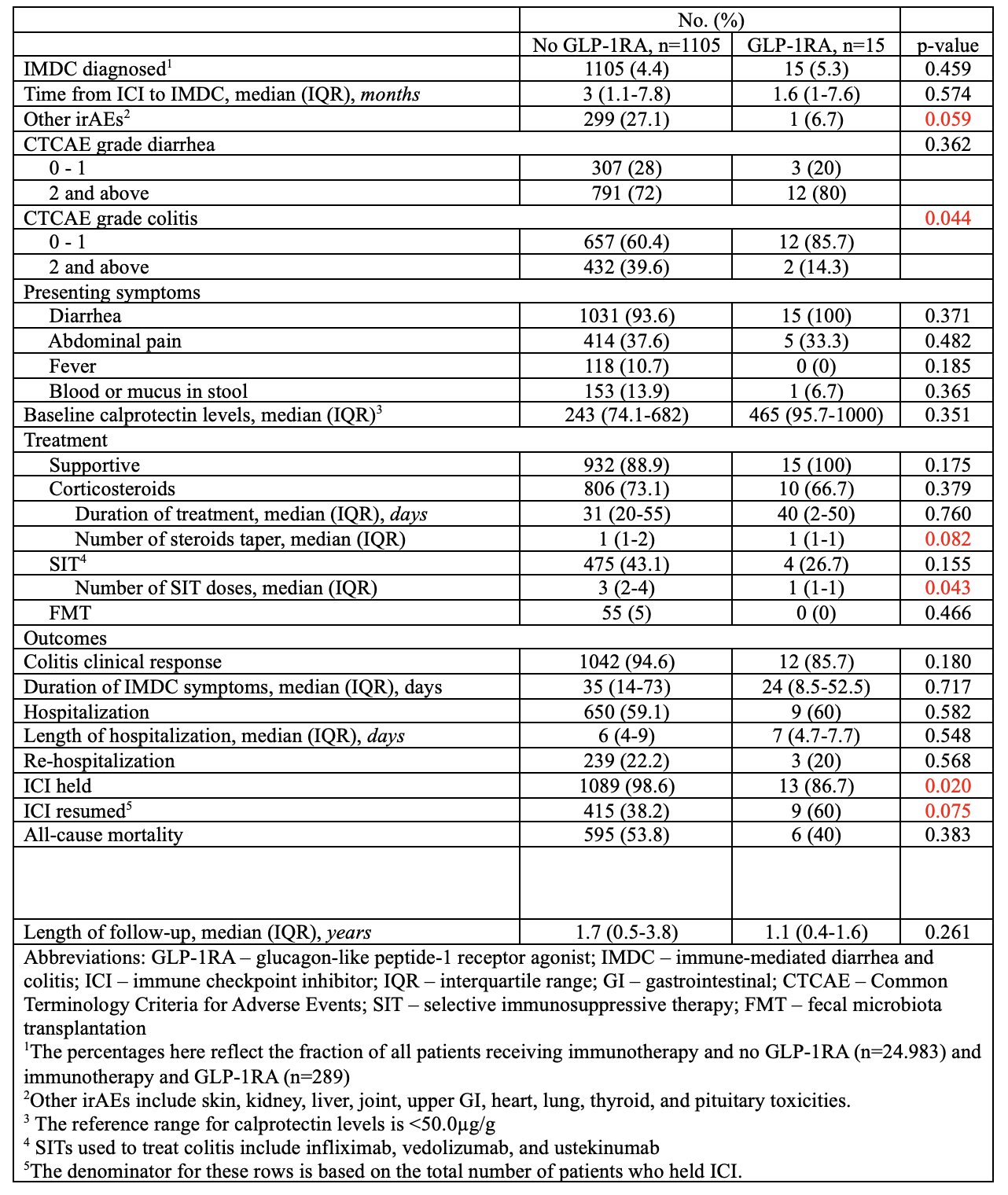
Among 1,128 patients, 58.6% were male and 89.8% white. Melanoma (34.9%) and genitourinary cancers (23.4%) were most common. ICI regimens included programmed cell death protein-1/ligand-1 inhibitors 553 (49%), cytotoxic T-lymphocyte-associated protein 4 inhibitors 192 (17%), and combined therapy 380 (33.6%). IMDC occurred in 5.3% of GLP-1RA (15) users vs 4.4% of non-users (1105). Despite similar biomarkers, GLP-1RA users more often had CTCAE grade< 2 colitis (85.7% vs 60.4%, p=0.044), required fewer SIT doses (1 vs 3, p=0.043), and trended toward fewer steroid tapers (1 vs. 1, p=0.082). ICI discontinuation was less common (86.7% vs 98.6%, p=0.020), and resumption more frequent (60% vs 38.2%, p=0.075). No significant mortality difference was observed. Compared to metformin users (IMDC incidence 7%, n=105), GLP-1RA users had milder colitis (CTCAE grade < 2: 85.7% vs 56.2%, p=0.030), higher ICI resumption (60% vs 31.4%, p=0.030), fewer SIT needs (1 vs 2, p=0.075), and lower mortality (40% vs 65.7%, p=0.054).
Discussion:
This is the first study to explore GLP-1RA use during ICI therapy. GLP-1RA users demonstrated milder IMDC, reduced immunosuppression needs, higher rates of ICI resumption, and better survival compared to metformin users. While not all outcomes reached statistical significance, consistent trends suggest a possible immunomodulatory benefit. These findings support further investigation into GLP-1RA as a potentially favorable alternative in patients with T2DM receiving ICIs.
Disclosures:
Cristina Natha, MD1, Carolina Cruz, MD2, Rohan Patel, 3, Anirudha Chatterjee, MD4, Varun Vemulapalli, MD1, Nina Quirk, MD, MS5, Rachel Mortan, MD6, Humberto R.. Nieves-Jiménez, MD7, Rohan Ahuja, MD6, Irene Lee, 8, Jacob Reitnauer, 6, Sean Ngo, BS1, Jarrett Rong, MD9, Tanvi Gupta, MD10, Reema Patel, MD4, Sharada Wali, MBBS, MPH2, Maria Julia M. N.. Santos, MD3, Krishnavathana Varatharajalu, MD3, Jessemel Estrada, MD4, Yinghong Wang, MD, PhD, MS2. P2450 - Reduced Immunosuppression Requirements With GLP-1 Receptor Agonists Use in Patients Experiencing Immune-Mediated Diarrhea and Colitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
