Monday Poster Session
Category: IBD
P3231 - Mirikizumab Demonstrates Rapid and Sustained Improvements in Bowel Urgency Measures and Clinical Measures in Moderately-to-Severely Active Ulcerative Colitis: 28-Week Results from the Phase 3b LUCENT-URGE Trial
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
Has Audio

Marla C. Dubinsky, MD
Susan and Leonard Feinstein IBD Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA
New York, NY
Presenting Author(s)
Silvio Danese, MD, PhD1, Axel Dignass, MD, PhD2, David Laharie, 3, Jimmy Limdi, MD4, Radoslaw Kempinski, MD, PhD5, James D. Lewis, MD6, Ziad Younes, MD7, Erica R. Cohen, MD8, Karla Alaka, 9, William J. Eastman, 9, Tian Tian, 9, Isabel Redondo, MD9, David T. Rubin, MD10, Marla C. Dubinsky, MD11
1Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milan, Lombardia, Italy; 2Agaplesion Markus Hospital, Frankfurt, Hessen, Germany; 3CHU de Bordeaux, Centre Medico-chirurgical Magellan, Hôpital Haut-Lévêque, Gastroenterology Department; Université de Bordeaux, Pessac, Aquitaine, France; 4Northern Care Alliance NHS Foundation Trust and University of Manchester, Manchester, England, United Kingdom; 5Wrocław Medical University, Wrocław, Lubelskie, Poland; 6Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA; 7Gastroenterology Center of the MidSouth PC, Germantown, Germantown, TN; 8Capital Digestive Care, Chevy Chase, Maryland, MD, USA, Chevy Chase, MD; 9Eli Lilly and Company, Indianapolis, IN; 10University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL; 11Susan and Leonard Feinstein IBD Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY
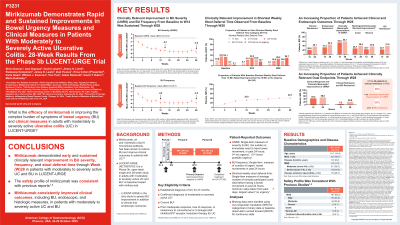
Introduction: LUCENT-URGE is a Phase 3b, open-label, single-arm 28-week (W) study in adults with moderately-to-severely active ulcerative colitis (UC) and bowel urgency (BU, Urgency Numeric Rating Scale [UNRS] ≥3) at baseline treated with mirikizumab (MIRI). Here, we report W28 results from LUCENT-URGE.
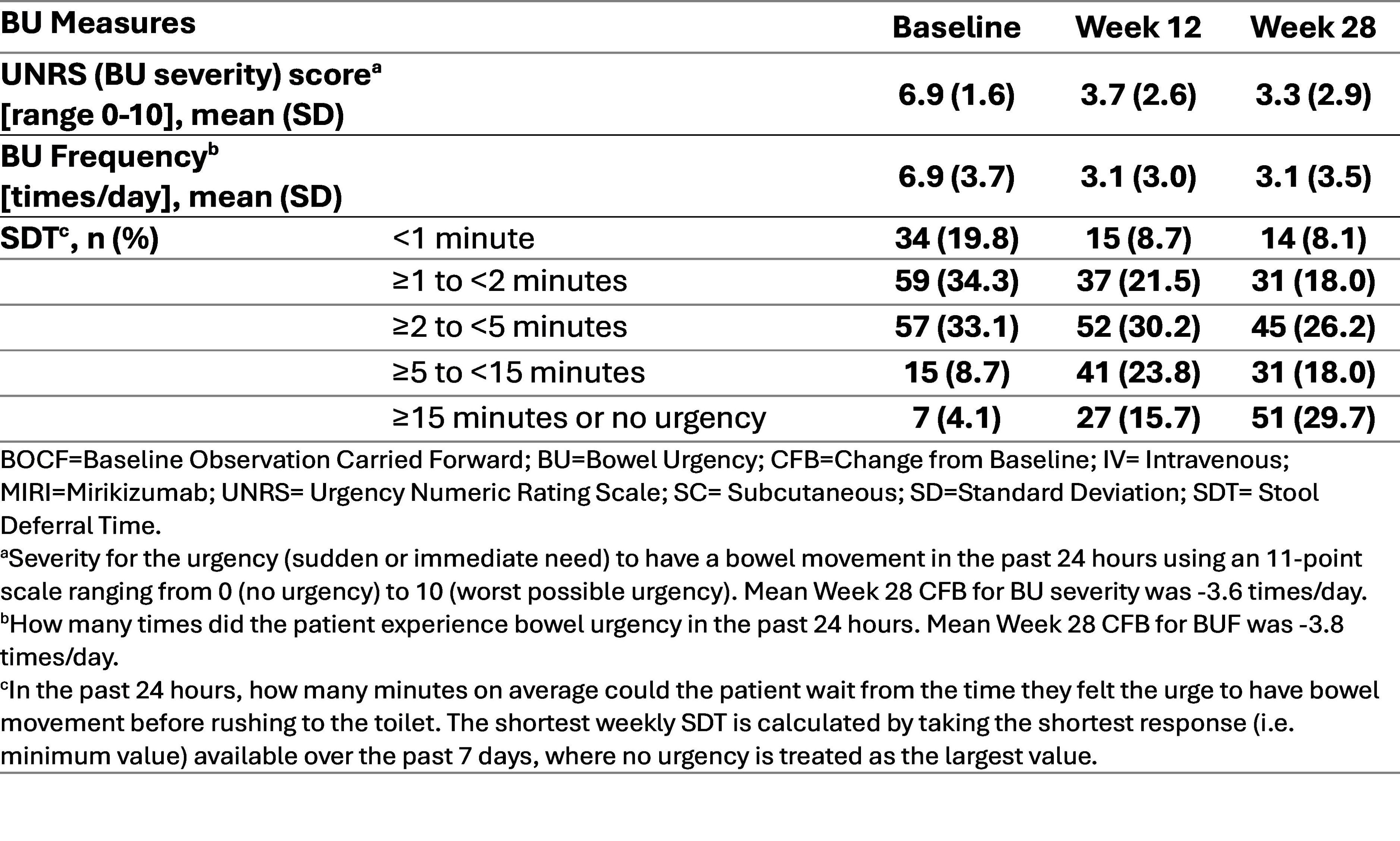
Methods: Patients (pts) received intravenous MIRI 300 mg at W0, 4 and 8, followed by 200 mg subcutaneous at W12, 16, 20, and 24. Primary objective: UNRS improvement at W12. Secondary objectives: UNRS improvement at W28, Stool Deferral Time (SDT) and Bowel Urgency Frequency (BUF) improvement at W12 and W28, proportion of participants achieving clinical remission and an UNRS score of ≤1 (BU remission) at W12 and W28, and those achieving clinical response and an improvement in UNRS score ≥3 (clinically meaningful improvement [CMI] of UNRS) at W12 and W28. UNRS and BUF were measured using a daily diary where pts recorded the number of times they experienced BU in the past 24 hours, averaged over a 7-day period. The shortest weekly SDT was used.
Results: 172 pts were enrolled from 8 countries. Baseline modified Mayo score (MMS; mean± standard deviation [SD]) was 6.8±1.3, and 64.0% pts had severe disease activity (MMS 7-9). Baseline mean duration of UC was 8.0 years, mean UNRS score was 6.9 and mean BUF was 6.9 times/day. Mean UNRS and BUF improved from baseline (3.6) to W28 (3.8). The proportion of pts with SDT defined as “≥15 minutes or no urgency” was 4.1% at baseline and improved to 29.7% at W28 (Table). W12: 36 (20.9%) pts achieved clinical remission, 54 (31.4%) achieved endoscopic remission, and 92 (53.5%) achieved CMI of UNRS. W28: 62 (36.1%) pts achieved clinical remission, 76 (44.2%) achieved endoscopic remission, and 100 (58.1%) achieved CMI of UNRS. 76 (44.2%) pts achieved both clinical response and CMI of UNRS at W12, and 86 (50.0%) pts at W28. W12 and W28: 8 patients (4.7%) and 28 patients (16.3%) achieved both clinical remission and BU remission, respectively. The safety profile of MIRI in LUCENT-URGE was consistent with previous studies, with no death reported and similar frequency of treatment-emergent adverse events (TEAEs). Most common TEAEs: ulcerative colitis exacerbation (5.2%) and upper respiratory tract infection (5.2%).
Discussion: MIRI showed an early and sustained improvement on BU severity, frequency and stool deferral time through W28 in LUCENT-URGE. MIRI consistently improved clinical outcomes in pts with moderately-to-severely active UC.
Disclosures:
Silvio Danese, MD, PhD1, Axel Dignass, MD, PhD2, David Laharie, 3, Jimmy Limdi, MD4, Radoslaw Kempinski, MD, PhD5, James D. Lewis, MD6, Ziad Younes, MD7, Erica R. Cohen, MD8, Karla Alaka, 9, William J. Eastman, 9, Tian Tian, 9, Isabel Redondo, MD9, David T. Rubin, MD10, Marla C. Dubinsky, MD11. P3231 - Mirikizumab Demonstrates Rapid and Sustained Improvements in Bowel Urgency Measures and Clinical Measures in Moderately-to-Severely Active Ulcerative Colitis: 28-Week Results from the Phase 3b LUCENT-URGE Trial, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milan, Lombardia, Italy; 2Agaplesion Markus Hospital, Frankfurt, Hessen, Germany; 3CHU de Bordeaux, Centre Medico-chirurgical Magellan, Hôpital Haut-Lévêque, Gastroenterology Department; Université de Bordeaux, Pessac, Aquitaine, France; 4Northern Care Alliance NHS Foundation Trust and University of Manchester, Manchester, England, United Kingdom; 5Wrocław Medical University, Wrocław, Lubelskie, Poland; 6Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA; 7Gastroenterology Center of the MidSouth PC, Germantown, Germantown, TN; 8Capital Digestive Care, Chevy Chase, Maryland, MD, USA, Chevy Chase, MD; 9Eli Lilly and Company, Indianapolis, IN; 10University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL; 11Susan and Leonard Feinstein IBD Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY
Introduction: LUCENT-URGE is a Phase 3b, open-label, single-arm 28-week (W) study in adults with moderately-to-severely active ulcerative colitis (UC) and bowel urgency (BU, Urgency Numeric Rating Scale [UNRS] ≥3) at baseline treated with mirikizumab (MIRI). Here, we report W28 results from LUCENT-URGE.
Methods: Patients (pts) received intravenous MIRI 300 mg at W0, 4 and 8, followed by 200 mg subcutaneous at W12, 16, 20, and 24. Primary objective: UNRS improvement at W12. Secondary objectives: UNRS improvement at W28, Stool Deferral Time (SDT) and Bowel Urgency Frequency (BUF) improvement at W12 and W28, proportion of participants achieving clinical remission and an UNRS score of ≤1 (BU remission) at W12 and W28, and those achieving clinical response and an improvement in UNRS score ≥3 (clinically meaningful improvement [CMI] of UNRS) at W12 and W28. UNRS and BUF were measured using a daily diary where pts recorded the number of times they experienced BU in the past 24 hours, averaged over a 7-day period. The shortest weekly SDT was used.
Results: 172 pts were enrolled from 8 countries. Baseline modified Mayo score (MMS; mean± standard deviation [SD]) was 6.8±1.3, and 64.0% pts had severe disease activity (MMS 7-9). Baseline mean duration of UC was 8.0 years, mean UNRS score was 6.9 and mean BUF was 6.9 times/day. Mean UNRS and BUF improved from baseline (3.6) to W28 (3.8). The proportion of pts with SDT defined as “≥15 minutes or no urgency” was 4.1% at baseline and improved to 29.7% at W28 (Table). W12: 36 (20.9%) pts achieved clinical remission, 54 (31.4%) achieved endoscopic remission, and 92 (53.5%) achieved CMI of UNRS. W28: 62 (36.1%) pts achieved clinical remission, 76 (44.2%) achieved endoscopic remission, and 100 (58.1%) achieved CMI of UNRS. 76 (44.2%) pts achieved both clinical response and CMI of UNRS at W12, and 86 (50.0%) pts at W28. W12 and W28: 8 patients (4.7%) and 28 patients (16.3%) achieved both clinical remission and BU remission, respectively. The safety profile of MIRI in LUCENT-URGE was consistent with previous studies, with no death reported and similar frequency of treatment-emergent adverse events (TEAEs). Most common TEAEs: ulcerative colitis exacerbation (5.2%) and upper respiratory tract infection (5.2%).
Discussion: MIRI showed an early and sustained improvement on BU severity, frequency and stool deferral time through W28 in LUCENT-URGE. MIRI consistently improved clinical outcomes in pts with moderately-to-severely active UC.

Figure: Change in Bowel Urgency Measures from Baseline at Week 12 and Week 28, BOCF
Disclosures:
Silvio Danese: AbbVie – Consultant, Lecture fees. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Lecture fees. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Celltrion – Consultant. Eli Lilly – Consultant. Enthera – Consultant. F. Hoffmann-La Roche Ltd – Consultant. Ferring Pharmaceuticals Inc. – Consultant, Lecture fees. Gilead – Consultant, Lecture fees. Hospira – Consultant. Inotrem – Consultant. Johnson & Johnson – Consultant, Lecture fees. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Lecture fees. Pfizer – Consultant, Lecture fees. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Lecture fees. TiGenix – Consultant. UCB Inc. – Consultant. Vifor (International) Ltd. – Consultant.
Axel Dignass: Abbvie – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and endpoint committees; manuscripts, Speakers Bureau. Abivax – fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees. Amgen – Consultant. Arena Pharmaceuticals – Consultant. Biogen – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. CED Service GmbH – Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Dr Falk Foundation – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and endpoint committees; manuscripts, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Galapagos – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Gilead – fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. High5MD – Speakers Bureau. Johnson & Johnson – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and endpoint committees; manuscripts, Speakers Bureau. Lilly – Consultant. Materia – Speakers Bureau. MedToday – Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Pharmacosmos – Consultant. Prima – Speakers Bureau. Roche – Consultant. Sandoz – Consultant. Stada – Consultant. Takeda – Consultant, manuscript preparation, Speakers Bureau. Thieme – manuscript preparation. Tilliots – Consultant, Speakers Bureau. UniMed Verlag – manuscript preparation. Vifor Pharma – Consultant, Speakers Bureau.
David Laharie: AbbVie – Advisory Committee/Board Member, Consultant, Transport, Fees.. Alfasigma – Advisory Committee/Board Member, Consultant, Transport or Fees. Amgen – Advisory Committee/Board Member, Consultant, Transport, Fees.. Celltrion – Advisory Committee/Board Member, Consultant, Transport, Fees.. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Transport, Fees.. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant, Transport, Fees.. Janssen – Advisory Committee/Board Member, Consultant, Transport, Fees.. Medac – Advisory Committee/Board Member, Consultant, Transport or Fees. Merck Sharp & Dohme – Advisory Committee/Board Member, Consultant, Transport, Fees.. Pfizer – Advisory Committee/Board Member, Consultant, Transport, Fees.. Prometheus – Advisory Committee/Board Member, Consultant, Transport, Fees.. Takeda – Advisory Committee/Board Member, Consultant, Transport, Fees.. Theradiag – Advisory Committee/Board Member, Consultant, Transport, Fees..
Jimmy Limdi: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Abivax – Consultant, Speakers Bureau. AlfaSigma – Consultant, Speakers Bureau. Biohit – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Eli Lilly and Company – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Grant/Research Support, Speakers Bureau. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau.
Radoslaw Kempinski: AbbVie – Lecture fees. Aboca S.p.A. Società Agricola – Lecture fees. Eli Lilly and Company – Lecture fees. Ferring – Lecture fees. Takeda – Grant/Research Support.
James D. Lewis: AbbVie – Grant/Research Support. Amgen – Advisory Committee/Board Member, Consultant, Data monitoring committee. Crohn’s & Colitis Foundation – Advisory Committee/Board Member, Consultant, Data monitoring committee. Dark Canyon Labs – Stock Options. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Grant/Research Support, Data monitoring committee. Galapagos – Advisory Committee/Board Member, Consultant, Data monitoring committee. Janssen Pharmaceuticals (Johnson & Johnson) – Advisory Committee/Board Member, Consultant, Grant/Research Support, Data monitoring committee, Educational grant. Merck – Advisory Committee/Board Member, Consultant, Data monitoring committee. Nestle Health Science – Grant/Research Support. Odyssey Therapeutics – Advisory Committee/Board Member, Consultant, Data monitoring committee. Pfizer – Advisory Committee/Board Member, Consultant, Data monitoring committee. Protagonist Therapeutics – Advisory Committee/Board Member, Consultant, Data monitoring committee. Sanofi – Advisory Committee/Board Member, Consultant, Data monitoring committee. Spyre Pharmaceuticals – Advisory Committee/Board Member, Consultant, Data monitoring committee. Takeda – Grant/Research Support.
Ziad Younes: AbbVie – Grant/Research Support, Speakers Bureau. Axcella – Grant/Research Support. Bristol Myers Squibb – Grant/Research Support, Speakers Bureau. CymaBay – Grant/Research Support. Galectin – Grant/Research Support. Intercept – Consultant, Grant/Research Support, Speakers Bureau. Inventiva – Grant/Research Support. Madrigal – Consultant, Grant/Research Support. NGM – Grant/Research Support. Novo Nordisk – Grant/Research Support. NST – Grant/Research Support. Poxel – Grant/Research Support. Sagimet – Grant/Research Support.
Erica Cohen: AbbVie – Speakers Bureau. Eli Lilly and Company – Speakers Bureau. Janssen – Speakers Bureau. Pfizer – Advisory Committee/Board Member, Consultant. PRIME Education – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Karla Alaka: Eli Lilly and Company – Employee, Stock Options.
William Eastman: Eli Lilly and Company – Employee, Stock Options.
Tian Tian: Eli Lilly and Company – Employee, Stock Options.
Isabel Redondo: Eli Lilly and Company – Employee, Stock Options.
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Marla Dubinsky: AbbVie – Consultant, Grant/Research Support. Arena – Consultant. Astra Zeneca – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Eli Lilly – Consultant. Gilead – Consultant. Janssen – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant. Prometheus Laboratories – Consultant, Grant/Research Support. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant. Trellus Health – Stock Options, Stock-publicly held company(excluding mutual/index funds). UCB – Consultant.
Silvio Danese, MD, PhD1, Axel Dignass, MD, PhD2, David Laharie, 3, Jimmy Limdi, MD4, Radoslaw Kempinski, MD, PhD5, James D. Lewis, MD6, Ziad Younes, MD7, Erica R. Cohen, MD8, Karla Alaka, 9, William J. Eastman, 9, Tian Tian, 9, Isabel Redondo, MD9, David T. Rubin, MD10, Marla C. Dubinsky, MD11. P3231 - Mirikizumab Demonstrates Rapid and Sustained Improvements in Bowel Urgency Measures and Clinical Measures in Moderately-to-Severely Active Ulcerative Colitis: 28-Week Results from the Phase 3b LUCENT-URGE Trial, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
