Monday Poster Session
Category: Infections and Microbiome
P3449 - Seven-Day Tegoprazan Therapy Matches Proton Pump Inhibitors and 14-Day Therapies for H. pylor Eradication With Much Fewer Adverse Events: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Ashesh Das, MBBS
KPC Medical College and Hospital , Kolkata, India
Kolkata, West Bengal, India
Presenting Author(s)
Award: ACG Presidential Poster Award
Ashesh Das, MBBS1, Venkata Dileep Kumar Veldi, MBBS2, Muqadas Bhatti, BS3, Yash Balubhai. Nasit, MBBS4, Sai VENKAT. Madduri, MBBS5, Anushka Anand. Hanchate, MBBS6, Sai Madhav Dongur, MD, MHA7, Srivatsa Anidruth Chelluri, 8, Shankar Biswas, MD9, Mahak Bhandari, MBBS10, Ahana Dutta, MBBS11, Ashfaq Sulaiman Arif Abdul Rahuman, MBBS12, Siddharth Samudram, 13
1KPC Medical College and Hospital , Kolkata, India, Kolkata, West Bengal, India; 2Gayatri Vidya Parishad Institute of Health care and Medical Technology, Visakhapatnam, Andhra Pradesh, India; 3Bahria University, Karachi, Pakistan, Karachi, Sindh, Pakistan; 4Government Medical College and Hospital, Surat, Surat, Gujarat, India; 5Andhra medical College, visakhapatnam, Rajahmundry, Andhra Pradesh, India; 6Mgm Medical College and Hopsital , Kamothe ,Navi mumbai, Mumbai, Maharashtra, India; 7University of Wisconsin Milwaukee, Milwaukee, WI; 8Osmania General Hospital and Medical College, Hyderabad, Telangana, India; 9Ivano-Frankivsk National Medical University, Ukraine, Meerut, Uttar Pradesh, India; 10Lokmanya Tilak Municipal Medical College, Mumbai, Maharashtra, India; 11KPC Medical College and Hospital, Kolkata, West Bengal, India; 12Madras Medical College, Thiruvananthapuram, Kerala, India; 13Osmania Medical College, Hyderabad, MD, India
Introduction: Despite guideline-mandated 14-day quadruple therapy, real-world eradication of Helicobacter pylori continues to falter because proton-pump inhibitors (PPIs) give slow, CYP2C19-dependent acid control and carry growing clarithromycin resistance. Potassium-competitive acid blockers such as Tegoprazan achieve near-instant, sustained pH > 6 and have delivered non-inferior or superior cure rates in trials while shortening therapy duration and mitigating dyspepsia-related toxicity. Whether a 7-day Tegoprazan-based course can match conventional PPI or longer Tegoprazan regimens and simultaneously improve tolerability remains a clinically pivotal question for stewardship.
Methods: A systematic search of PubMed, Embase, Scopus, and Cochrane Library identified Randomized Controlled Trials (RCTs) comparing 7 Day Tegoprazan Therapy versus PPIs or 14 Day Regimens for H pylori eradication through May 2025. Data were analysed using RevMan 4.2.1. Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using Mantel-Haenszel methods. Random- or fixed-effects models were applied based on heterogeneity (Higgins’ I²). Statistical significance was set at p < 0.05. Risk of bias was assessed using RoB 2.0.
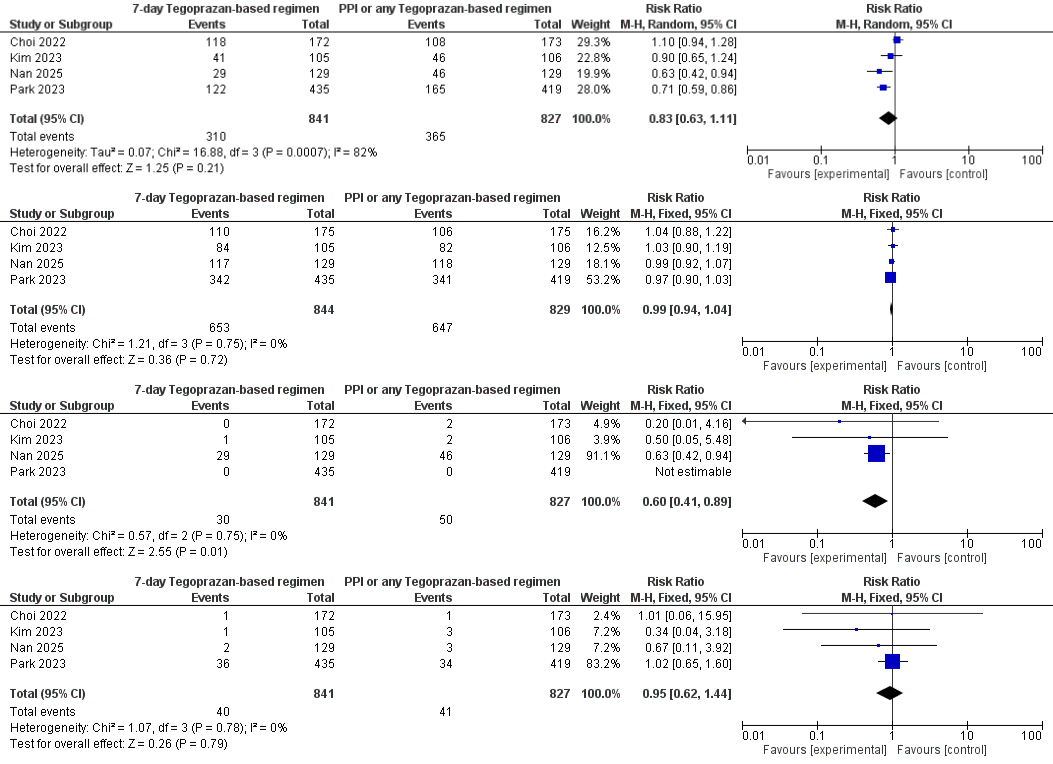
Results: Four RCTs (N = 1,668; 7-day Tegoprazan = 841, control = 827) showed identical eradication efficacy confirmed by urea breath test (RR 0.99, 95% CI 0.94-1.04; I² = 0%) with the abbreviated Tegoprazan regimen. Serious adverse events (SAEs) fell by 40% (RR 0.60, 95% CI 0.41-0.89; I² = 0%), translating to 1 fewer SAE per ~ 43 patients treated. Overall adverse-event rates were numerically lower (RR 0.83, 95% CI 0.63-1.11) but heterogeneous (I² = 82%), while treatment discontinuation remained comparable (RR 0.95, 95% CI 0.62-1.44; I² = 0%).
Discussion: Rapid, potent acid suppression allows a 7-day Tegoprazan backbone to deliver guideline-level cure rates while halving serious toxicity versus PPI or longer PCAB courses—an advantage likely driven by briefer antibiotic exposure and gastro-intestinal neutrality of Tegoprazan. The negligible heterogeneity for key endpoints and consistent benefit support adoption. High dispersion in any adverse event signals variable adverse events reporting rather than biological inconsistency. Clinically, this regimen offers a streamlined, patient-friendly, cost-aware alternative that aligns with antimicrobial-stewardship goals and may improve global uptake of H. pylori eradication.
Disclosures:
Ashesh Das, MBBS1, Venkata Dileep Kumar Veldi, MBBS2, Muqadas Bhatti, BS3, Yash Balubhai. Nasit, MBBS4, Sai VENKAT. Madduri, MBBS5, Anushka Anand. Hanchate, MBBS6, Sai Madhav Dongur, MD, MHA7, Srivatsa Anidruth Chelluri, 8, Shankar Biswas, MD9, Mahak Bhandari, MBBS10, Ahana Dutta, MBBS11, Ashfaq Sulaiman Arif Abdul Rahuman, MBBS12, Siddharth Samudram, 13. P3449 - Seven-Day Tegoprazan Therapy Matches Proton Pump Inhibitors and 14-Day Therapies for <i>H. pylor</i> Eradication With Much Fewer Adverse Events: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Ashesh Das, MBBS1, Venkata Dileep Kumar Veldi, MBBS2, Muqadas Bhatti, BS3, Yash Balubhai. Nasit, MBBS4, Sai VENKAT. Madduri, MBBS5, Anushka Anand. Hanchate, MBBS6, Sai Madhav Dongur, MD, MHA7, Srivatsa Anidruth Chelluri, 8, Shankar Biswas, MD9, Mahak Bhandari, MBBS10, Ahana Dutta, MBBS11, Ashfaq Sulaiman Arif Abdul Rahuman, MBBS12, Siddharth Samudram, 13
1KPC Medical College and Hospital , Kolkata, India, Kolkata, West Bengal, India; 2Gayatri Vidya Parishad Institute of Health care and Medical Technology, Visakhapatnam, Andhra Pradesh, India; 3Bahria University, Karachi, Pakistan, Karachi, Sindh, Pakistan; 4Government Medical College and Hospital, Surat, Surat, Gujarat, India; 5Andhra medical College, visakhapatnam, Rajahmundry, Andhra Pradesh, India; 6Mgm Medical College and Hopsital , Kamothe ,Navi mumbai, Mumbai, Maharashtra, India; 7University of Wisconsin Milwaukee, Milwaukee, WI; 8Osmania General Hospital and Medical College, Hyderabad, Telangana, India; 9Ivano-Frankivsk National Medical University, Ukraine, Meerut, Uttar Pradesh, India; 10Lokmanya Tilak Municipal Medical College, Mumbai, Maharashtra, India; 11KPC Medical College and Hospital, Kolkata, West Bengal, India; 12Madras Medical College, Thiruvananthapuram, Kerala, India; 13Osmania Medical College, Hyderabad, MD, India
Introduction: Despite guideline-mandated 14-day quadruple therapy, real-world eradication of Helicobacter pylori continues to falter because proton-pump inhibitors (PPIs) give slow, CYP2C19-dependent acid control and carry growing clarithromycin resistance. Potassium-competitive acid blockers such as Tegoprazan achieve near-instant, sustained pH > 6 and have delivered non-inferior or superior cure rates in trials while shortening therapy duration and mitigating dyspepsia-related toxicity. Whether a 7-day Tegoprazan-based course can match conventional PPI or longer Tegoprazan regimens and simultaneously improve tolerability remains a clinically pivotal question for stewardship.
Methods: A systematic search of PubMed, Embase, Scopus, and Cochrane Library identified Randomized Controlled Trials (RCTs) comparing 7 Day Tegoprazan Therapy versus PPIs or 14 Day Regimens for H pylori eradication through May 2025. Data were analysed using RevMan 4.2.1. Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using Mantel-Haenszel methods. Random- or fixed-effects models were applied based on heterogeneity (Higgins’ I²). Statistical significance was set at p < 0.05. Risk of bias was assessed using RoB 2.0.
Results: Four RCTs (N = 1,668; 7-day Tegoprazan = 841, control = 827) showed identical eradication efficacy confirmed by urea breath test (RR 0.99, 95% CI 0.94-1.04; I² = 0%) with the abbreviated Tegoprazan regimen. Serious adverse events (SAEs) fell by 40% (RR 0.60, 95% CI 0.41-0.89; I² = 0%), translating to 1 fewer SAE per ~ 43 patients treated. Overall adverse-event rates were numerically lower (RR 0.83, 95% CI 0.63-1.11) but heterogeneous (I² = 82%), while treatment discontinuation remained comparable (RR 0.95, 95% CI 0.62-1.44; I² = 0%).
Discussion: Rapid, potent acid suppression allows a 7-day Tegoprazan backbone to deliver guideline-level cure rates while halving serious toxicity versus PPI or longer PCAB courses—an advantage likely driven by briefer antibiotic exposure and gastro-intestinal neutrality of Tegoprazan. The negligible heterogeneity for key endpoints and consistent benefit support adoption. High dispersion in any adverse event signals variable adverse events reporting rather than biological inconsistency. Clinically, this regimen offers a streamlined, patient-friendly, cost-aware alternative that aligns with antimicrobial-stewardship goals and may improve global uptake of H. pylori eradication.

Figure: Forest Plot Showing Eradication Rate (Confirmed by Urea Breath Test), Adverse Events, Serious Adverse Events and Discontinuation Rates
Disclosures:
Ashesh Das indicated no relevant financial relationships.
Venkata Dileep Kumar Veldi indicated no relevant financial relationships.
Muqadas Bhatti indicated no relevant financial relationships.
Yash Nasit indicated no relevant financial relationships.
Sai Madduri indicated no relevant financial relationships.
Anushka Hanchate indicated no relevant financial relationships.
Sai Madhav Dongur indicated no relevant financial relationships.
Srivatsa Anidruth Chelluri indicated no relevant financial relationships.
Shankar Biswas indicated no relevant financial relationships.
Mahak Bhandari indicated no relevant financial relationships.
Ahana Dutta indicated no relevant financial relationships.
Ashfaq Sulaiman Arif Abdul Rahuman indicated no relevant financial relationships.
Siddharth Samudram indicated no relevant financial relationships.
Ashesh Das, MBBS1, Venkata Dileep Kumar Veldi, MBBS2, Muqadas Bhatti, BS3, Yash Balubhai. Nasit, MBBS4, Sai VENKAT. Madduri, MBBS5, Anushka Anand. Hanchate, MBBS6, Sai Madhav Dongur, MD, MHA7, Srivatsa Anidruth Chelluri, 8, Shankar Biswas, MD9, Mahak Bhandari, MBBS10, Ahana Dutta, MBBS11, Ashfaq Sulaiman Arif Abdul Rahuman, MBBS12, Siddharth Samudram, 13. P3449 - Seven-Day Tegoprazan Therapy Matches Proton Pump Inhibitors and 14-Day Therapies for <i>H. pylor</i> Eradication With Much Fewer Adverse Events: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

