Tuesday Poster Session
Category: Endoscopy Video Forum
P4868 - Primary Hemostasis of a Bleeding Duodenal Ulcer Using Helical Tacking System in the Setting of Esophageal Stricture

Natalie Rosseau, MD
University of Florida Health Shands Hospital
Gainesville, FL
Presenting Author(s)
1University of Florida, Gainesville, FL; 2University of Florida Health Shands Hospital, Gainesville, FL; 3University of Florida College of Medicine, Gainesville, FL
Introduction:
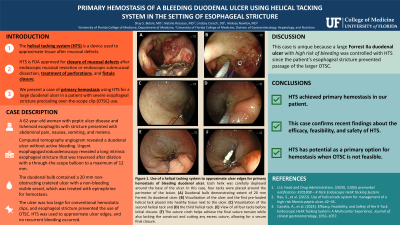
The helical tacking system (HTS) is a device used to approximate tissue after mucosal defects. It is FDA approved for closure of mucosal defects after endoscopic mucosal resection or endoscopic submucosal dissection, treatment of perforations, and fistula closure. It has also been reported as useful in hemostasis.1,2 We present a case of primary hemostasis using HTS for a large duodenal ulcer in a patient with severe esophageal stricture precluding over-the-scope clip (OTSC) use.
Case Description/Methods:
A 62-year-old woman with peptic ulcer disease and lichenoid esophagitis with stricture presented with abdominal pain, nausea, vomiting, and melena. She was hypotensive and admitted to the medical intensive care unit. Computed tomography angiogram revealed a duodenal ulcer without active bleeding. Urgent esophagogastroduodenoscopy revealed a long intrinsic esophageal stricture that was traversed after dilation with a through the scope balloon to a maximum of 11-12 mm. Duodenal bulb contained a 20 mm non-obstructing cratered ulcer with a non-bleeding visible vessel and treated with 5 mL of 0.1 mg/mL epinephrine for hemostasis. The ulcer was too large for conventional hemostatic clips and esophageal stricture prevented use of OTSC, so HTS was used to approximate ulcer edges. The patient tolerated the procedure well without recurrent bleeding. Biopsies were negative for H. pylori. Immediate inpatient treatment involved a clear liquid diet, IV pantoprazole 40 mg twice per day, and nonsteroidal anti-inflammatory drug restriction. She was discharged on omeprazole 20 mg daily.
Discussion:
This case is unique because a large Forrest IIa duodenal ulcer with high risk of bleeding was controlled with HTS because the patient’s esophageal stricture prevented passage of the larger OTSC. Though not approved for acutely bleeding ulcers, HTS achieved primary hemostasis in our case. This confirms recent findings from a retrospective study on HTS efficacy, feasibility, and safety. 3 Our case illustrates the potential of HTS as a viable primary option when OTSC is not feasible.
References
1. U.S. Food and Drug Administration. (2020). 510(k) premarket notification: K201808 – X-Tack Endoscopic HeliX Tacking System.
2. Rau, S., et al. (2022). Use of helical tack system for management of a high-risk fibrotic peptic ulcer, 42–45.
3. Canakis, A., et al. (2024). Efficacy, Feasibility, and Safety of the X-Tack Endoscopic HeliX Tacking System: A Multicenter Experience. Journal of clinical gastroenterology, 1052–1057.
Disclosures:
Shay Bidani, MD1, Natalie Rosseau, MD2, Lindsey A. Creech, DO, MBA, MPH1, Aleksey Novikov, MD3. P4868 - Primary Hemostasis of a Bleeding Duodenal Ulcer Using Helical Tacking System in the Setting of Esophageal Stricture, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
