Tuesday Poster Session
Category: Functional Bowel Disease
P5096 - Linaclotide’s Time to Response for Chronic Idiopathic Constipation Symptom Relief: A Post Hoc Analysis of Randomized Controlled Studies
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Has Audio

Darren M. Brenner, MD, FACG
Professor of Medicine and Surgery
Northwestern University Feinberg School of Medicine, Chicago, IL, US
Chicago, IL
Presenting Author(s)
Darren M.. Brenner, MD1, Brian E. Lacy, MD, PhD, FACG2, Charlotte C. Dancey, PhD3, Niha Yerneni, PharmD, MPH4, Moming Li, PhD3, Lin Chang, MD5
1Northwestern University Feinberg School of Medicine, Chicago, IL, US, Chicago, IL; 2Mayo Clinic, Jacksonville, FL, US, Jacksonville, FL; 3AbbVie Inc., North Chicago, IL, US, Chicago, IL; 4Ironwood Pharmaceuticals, Inc., Boston, MA, US, Boston, MA; 5Vatche and Tamar Manoukian Division of Digestive Diseases, David Geffen School of Medicine, UCLA, Los Angeles, CA, US, Los Angeles, CA
Introduction: Linaclotide (LIN) is FDA-approved to treat chronic idiopathic constipation (CIC) in adults. The efficacy of LIN for relieving bowel and abdominal CIC symptoms is established, but data on the timing of symptom relief are limited. This post hoc analysis examined the time to response for key symptoms of CIC in patients receiving LIN.
Methods: Data were pooled from four Phase 3 randomized studies (NCT00730015, NCT00765882, NCT01642914, NCT02291679) of adults with CIC who received LIN 72 µg, LIN 145 µg or placebo (PBO). All studies enrolled patients meeting modified Rome II/III criteria for CIC. Kaplan–Meier analyses assessed time to event (symptom relief) over 12 weeks. Bowel symptom relief was measured by complete spontaneous bowel movement (CSBM) response, defined as achieving ≥ 3 CSBMs/week, and first spontaneous bowel movement (SBM) after the initial treatment dose. Abdominal symptom relief was defined as a ≥ 30% improvement from baseline in weekly average score.
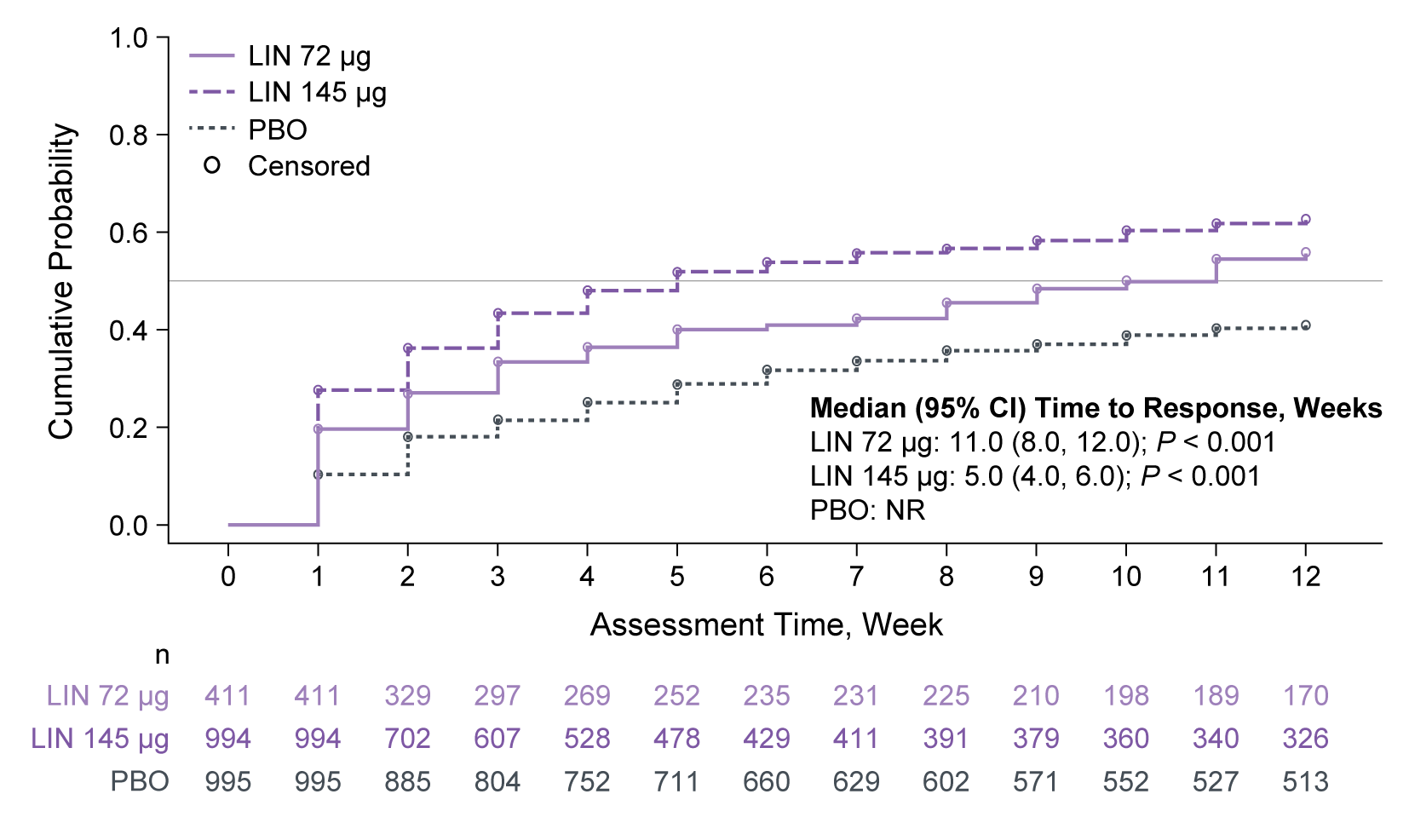
Results: Overall, 2400 patients were included: LIN 72 µg, n = 411, mean age: 45.8 years, female: 75.9%; LIN 145 µg, n = 994, 47.5 years, 84.3%; PBO, n = 995, 46.7 years, 85.4%. Median (95% CI) time to achieving ≥ 3 CSBMs/week was 11 (8, 12) and 5 (4, 6) weeks for patients treated with LIN 72 µg and 145 µg, respectively (not reached [NR] for PBO; Figure). Median time to first SBM after initial dose of LIN (72 µg or 145 µg) was 2 days vs 3 days with PBO. For abdominal symptoms, median time to response with LIN 72 µg and 145 µg, respectively, was NR and 8 weeks (pain), and 12 and 7 weeks (discomfort and bloating); all responses were NR for PBO. Based on median response times for each dose, patients were classified as ‘early’ (≤ median), ‘late’ ( > median) and ‘non’ (NR by 12 weeks) responders. CSBM and abdominal symptom responders were cross tabulated to explore response patterns (Table). Patients were most often both early CSBM responders and early abdominal symptom responders. A lack of improvement in one symptom did not rule out a response for the other.
Discussion: Compared with PBO, LIN reduced the median time to bowel symptom relief in adults with CIC; for the LIN 145 µg dose, most patients had CSBM improvements within 5 weeks. Further, time to relief of abdominal bloating and discomfort, common CIC symptoms, were improved with both LIN doses. These results reinforce the therapeutic value of LIN in addressing multifaceted symptom burden of CIC. Future research could explore the characteristics of early, late and nonresponders.

Disclosures:
Darren M.. Brenner, MD1, Brian E. Lacy, MD, PhD, FACG2, Charlotte C. Dancey, PhD3, Niha Yerneni, PharmD, MPH4, Moming Li, PhD3, Lin Chang, MD5. P5096 - Linaclotide’s Time to Response for Chronic Idiopathic Constipation Symptom Relief: A <i>Post Hoc</i> Analysis of Randomized Controlled Studies, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Northwestern University Feinberg School of Medicine, Chicago, IL, US, Chicago, IL; 2Mayo Clinic, Jacksonville, FL, US, Jacksonville, FL; 3AbbVie Inc., North Chicago, IL, US, Chicago, IL; 4Ironwood Pharmaceuticals, Inc., Boston, MA, US, Boston, MA; 5Vatche and Tamar Manoukian Division of Digestive Diseases, David Geffen School of Medicine, UCLA, Los Angeles, CA, US, Los Angeles, CA
Introduction: Linaclotide (LIN) is FDA-approved to treat chronic idiopathic constipation (CIC) in adults. The efficacy of LIN for relieving bowel and abdominal CIC symptoms is established, but data on the timing of symptom relief are limited. This post hoc analysis examined the time to response for key symptoms of CIC in patients receiving LIN.
Methods: Data were pooled from four Phase 3 randomized studies (NCT00730015, NCT00765882, NCT01642914, NCT02291679) of adults with CIC who received LIN 72 µg, LIN 145 µg or placebo (PBO). All studies enrolled patients meeting modified Rome II/III criteria for CIC. Kaplan–Meier analyses assessed time to event (symptom relief) over 12 weeks. Bowel symptom relief was measured by complete spontaneous bowel movement (CSBM) response, defined as achieving ≥ 3 CSBMs/week, and first spontaneous bowel movement (SBM) after the initial treatment dose. Abdominal symptom relief was defined as a ≥ 30% improvement from baseline in weekly average score.
Results: Overall, 2400 patients were included: LIN 72 µg, n = 411, mean age: 45.8 years, female: 75.9%; LIN 145 µg, n = 994, 47.5 years, 84.3%; PBO, n = 995, 46.7 years, 85.4%. Median (95% CI) time to achieving ≥ 3 CSBMs/week was 11 (8, 12) and 5 (4, 6) weeks for patients treated with LIN 72 µg and 145 µg, respectively (not reached [NR] for PBO; Figure). Median time to first SBM after initial dose of LIN (72 µg or 145 µg) was 2 days vs 3 days with PBO. For abdominal symptoms, median time to response with LIN 72 µg and 145 µg, respectively, was NR and 8 weeks (pain), and 12 and 7 weeks (discomfort and bloating); all responses were NR for PBO. Based on median response times for each dose, patients were classified as ‘early’ (≤ median), ‘late’ ( > median) and ‘non’ (NR by 12 weeks) responders. CSBM and abdominal symptom responders were cross tabulated to explore response patterns (Table). Patients were most often both early CSBM responders and early abdominal symptom responders. A lack of improvement in one symptom did not rule out a response for the other.
Discussion: Compared with PBO, LIN reduced the median time to bowel symptom relief in adults with CIC; for the LIN 145 µg dose, most patients had CSBM improvements within 5 weeks. Further, time to relief of abdominal bloating and discomfort, common CIC symptoms, were improved with both LIN doses. These results reinforce the therapeutic value of LIN in addressing multifaceted symptom burden of CIC. Future research could explore the characteristics of early, late and nonresponders.

Figure: Figure. Kaplan–Meier analysis of time to achieve ≥ 3 CSBMs/week.
n is the number of patients at risk in the intention-to-treat population (randomized patients who received ≥ 1 dose of study drug, with analysis values at both baseline and during the 12-week treatment period). An event was defined as the first week a patient achieved ≥ 3 CSBMs/week.
P values are descriptive only and calculated without any multiplicity adjustment.
CI, confidence interval; CSBM, complete spontaneous bowel movement; LIN, linaclotide; NR, not reached; PBO, placebo.
n is the number of patients at risk in the intention-to-treat population (randomized patients who received ≥ 1 dose of study drug, with analysis values at both baseline and during the 12-week treatment period). An event was defined as the first week a patient achieved ≥ 3 CSBMs/week.
P values are descriptive only and calculated without any multiplicity adjustment.
CI, confidence interval; CSBM, complete spontaneous bowel movement; LIN, linaclotide; NR, not reached; PBO, placebo.

Figure: Table. Contingency table showing the number and proportion of patients classified as early, late and nonresponders to treatment.
*Response for individual abdominal symptoms was defined as a ≥ 30% improvement from baseline in weekly average score. For patients who received LIN 72 μg, early responders were those with a response in ≤ 12 weeks for abdominal discomfort and bloating, and NR for abdominal pain. For patients who received LIN 145 μg, early responders were those with a response in ≤ 8 weeks for abdominal pain, and in ≤ 7 weeks for abdominal discomfort and bloating. Abdominal pain, abdominal discomfort and abdominal bloating were evaluated daily (at their worst) using either a 5-point or 11-point numerical rating scale depending on the clinical study; before pooling the data, abdominal symptom measurements were standardized by converting the 11-point scale to the 5-point scale.
Data represent the intention-to-treat population (randomized patients who received ≥ 1 dose of study drug, with analysis values at both baseline and during the 12-week treatment period).
CSBM, complete spontaneous bowel movement; LIN, linaclotide; NR, not reached.
*Response for individual abdominal symptoms was defined as a ≥ 30% improvement from baseline in weekly average score. For patients who received LIN 72 μg, early responders were those with a response in ≤ 12 weeks for abdominal discomfort and bloating, and NR for abdominal pain. For patients who received LIN 145 μg, early responders were those with a response in ≤ 8 weeks for abdominal pain, and in ≤ 7 weeks for abdominal discomfort and bloating. Abdominal pain, abdominal discomfort and abdominal bloating were evaluated daily (at their worst) using either a 5-point or 11-point numerical rating scale depending on the clinical study; before pooling the data, abdominal symptom measurements were standardized by converting the 11-point scale to the 5-point scale.
Data represent the intention-to-treat population (randomized patients who received ≥ 1 dose of study drug, with analysis values at both baseline and during the 12-week treatment period).
CSBM, complete spontaneous bowel movement; LIN, linaclotide; NR, not reached.
Disclosures:
Darren Brenner: Alnylam Pharmaceuticals – Advisor or Review Panel Member, Consultant, Speaker. Anji Pharma, Ardelyx – Advisor or Review Panel Member. Ardelyx, AbbVie, Ironwood Pharmaceuticals, Bayer, Blueprint Medicines, CinPhloro Pharma, Dr Reddy’s Laboratories, Gemelli Biotech, Laborie – Consultant. Ardelyx, AbbVie, Ironwood Pharmaceuticals, Salix Pharmaceuticals – Speakers Bureau. Entrinsic Bioscience – Advisor or Review Panel Member, Consultant, Speaker. International Foundation for GI Disorders – Advisory Committee/Board Member. Mahana Therapeutics, Owlstone Medical, Salix Pharmaceuticals, Vibrant Pharma – Consultant. Takeda Pharmaceuticals – Advisor or Review Panel Member, Consultant, Speaker. Vibrant Gastro – Advisor or Review Panel Member, Consultant, Speaker.
Brian Lacy: AbbVie – Grant/Research Support. Ardelyx – Consultant. Gemelli Biotech – Consultant. GSK – Consultant. Ironwood Pharmaceuticals – Consultant, Grant/Research Support.
Charlotte Dancey: AbbVie – Employee, Stock-publicly held company(excluding mutual/index funds).
Niha Yerneni: Ironwood Pharmaceuticals – Employee, Stock-publicly held company(excluding mutual/index funds).
Moming Li: AbbVie – Employee, Stock-publicly held company(excluding mutual/index funds).
Lin Chang: AnX Robotica – Grant/Research Support. Ardelyx – Advisory Committee/Board Member. Atmo BioSciences – Advisory Committee/Board Member. Bausch Health – Consultant. FoodMarble Digestive Health – Consultant, Stock Options. GlaxoSmithKline – Consultant. Ironwood Pharmaceuticals – Advisory Committee/Board Member, Grant/Research Support. Lilly – Consultant. ModifyHealth – Stock Options. Trellus Health – Consultant, Stock Options. Vibrant Gastro – Advisory Committee/Board Member.
Darren M.. Brenner, MD1, Brian E. Lacy, MD, PhD, FACG2, Charlotte C. Dancey, PhD3, Niha Yerneni, PharmD, MPH4, Moming Li, PhD3, Lin Chang, MD5. P5096 - Linaclotide’s Time to Response for Chronic Idiopathic Constipation Symptom Relief: A <i>Post Hoc</i> Analysis of Randomized Controlled Studies, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
