Tuesday Poster Session
Category: IBD
P5479 - Mirikizumab Long-Term Impact on Bowel Urgency in Crohn’s Disease and Its Association With Clinical and Other Efficacy Outcomes: Results From the VIVID-2 Open-Label Extension Study
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
Has Audio
- JW
Jianmin Wu
Eli Lilly and Company
Indianapolis, IN
Presenting Author(s)
Stephan Vavricka, MD1, David T. Rubin, MD2, Jianmin Wu, 3, Guanglei Yu, PhD4, Aisha Vadhariya, 3, Richard E.. Moses, DO, JD3, Rebecca Hozak, 3, Geert R. D’Haens, MD, PhD5, Simon Travis, 6, Alissa Walsh, MBBS, PhD6
1Department of Gastroenterology and Hepatology, University Hospital Zürich, Zürich, Zurich, Switzerland; 2University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL; 3Eli Lilly and Company, Indianapolis, IN; 4Eli Lilly and Company, Indianapolis, Indiana, USA, Indianapolis, IN; 5Department of Gastroenterology, Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 6Translational Gastroenterology Unit, University of Oxford and Oxford Biomedical Research Centre, Oxford, UK, Oxford, England, United Kingdom
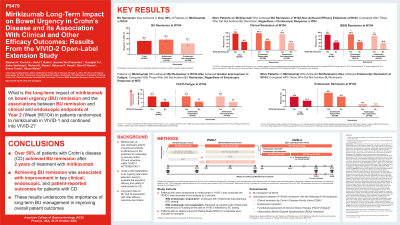
Introduction: Long-term data on bowel urgency (BU) in Crohn’s disease (CD) are limited, and the associations with other efficacy outcomes are not well studied.
Methods: To examine the long-term efficacy of mirikizumab (MIRI) on BU remission and the associations between BU remission and clinical remission, endoscopic remission, and patient-reported outcomes(PRO) improvement at week(W) 104 in patients(pts) with moderately to severely active CD who were randomized to MIRI in the Phase 3 study VIVID-1 and continued into the long-term extension study VIVID-2. In VIVID-1, the MIRI group received induction with 900 mg intravenously (IV) at W0, W4, and W8, then 300 mg subcutaneously (SC) every 4W. W52 endoscopic responders (≥50% reduction from baseline in Simple Endoscopic Score for CD[SES-CD]) continued MIRI SC dosing in VIVID-2. Endoscopic non-responders received reinduction with MIRI IV (3x Q4W) at the start of VIVID-2 followed by SC dosing (IV-SC) as described. Pts having an Urgency Numeric Rating Scale (UNRS) ≥3 at baseline were included in these analyses. BU remission was defined as an UNRS score of ≤2. Associations between BU remission with clinical remission by Crohn’s Disease Activity Index (CDAI), endoscopic remission, Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue, and Inflammatory Bowel Disease Questionnaire (IBDQ) remission, were assessed at W104, within the VIVID-1 W52 endoscopic response subgroups and the MIRI total group.
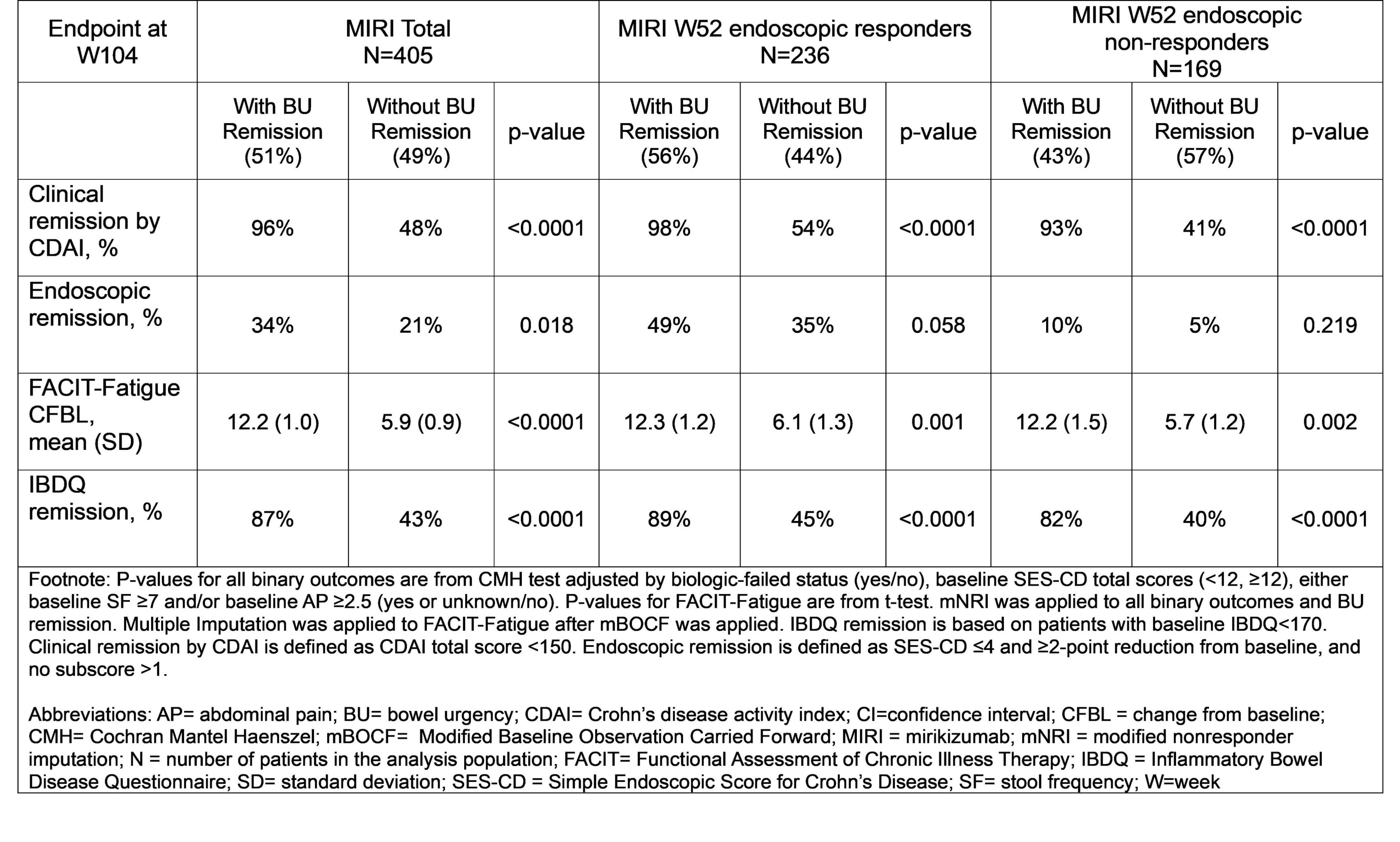
Results: Over 50% of the MIRI total group, 56% MIRI W52 endoscopic responders, and 43% MIRI W52 endoscopic non-responders achieved W104 BU remission. In the MIRI total group, a significantly higher percentage of pts achieved clinical remission, endoscopic remission and IBDQ remission at W104 in the BU remission group vs those without BU remission. The improvement in fatigue by FACIT-Fatigue score was significantly more in the group with BU remission vs those without BU remission (p< 0.0001). In both MIRI W52 endoscopic responders and non-responders, pts who achieved W104 BU remission, vs who did not, achieved significantly higher rates of clinical remission, greater improvements in FACIT-Fatigue, and higher rates of quality of life improvement measured by IBDQ remission (Table).
Discussion: Over 50% pts with CD achieved BU remission at 2 years of MIRI treatment. Achieving BU remission corresponded with improvements to outcomes important to pts with CD, underscoring the importance of long-term management of BU in improving overall pt outcomes.
Disclosures:
Stephan Vavricka, MD1, David T. Rubin, MD2, Jianmin Wu, 3, Guanglei Yu, PhD4, Aisha Vadhariya, 3, Richard E.. Moses, DO, JD3, Rebecca Hozak, 3, Geert R. D’Haens, MD, PhD5, Simon Travis, 6, Alissa Walsh, MBBS, PhD6. P5479 - Mirikizumab Long-Term Impact on Bowel Urgency in Crohn’s Disease and Its Association With Clinical and Other Efficacy Outcomes: Results From the VIVID-2 Open-Label Extension Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Department of Gastroenterology and Hepatology, University Hospital Zürich, Zürich, Zurich, Switzerland; 2University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL; 3Eli Lilly and Company, Indianapolis, IN; 4Eli Lilly and Company, Indianapolis, Indiana, USA, Indianapolis, IN; 5Department of Gastroenterology, Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 6Translational Gastroenterology Unit, University of Oxford and Oxford Biomedical Research Centre, Oxford, UK, Oxford, England, United Kingdom
Introduction: Long-term data on bowel urgency (BU) in Crohn’s disease (CD) are limited, and the associations with other efficacy outcomes are not well studied.
Methods: To examine the long-term efficacy of mirikizumab (MIRI) on BU remission and the associations between BU remission and clinical remission, endoscopic remission, and patient-reported outcomes(PRO) improvement at week(W) 104 in patients(pts) with moderately to severely active CD who were randomized to MIRI in the Phase 3 study VIVID-1 and continued into the long-term extension study VIVID-2. In VIVID-1, the MIRI group received induction with 900 mg intravenously (IV) at W0, W4, and W8, then 300 mg subcutaneously (SC) every 4W. W52 endoscopic responders (≥50% reduction from baseline in Simple Endoscopic Score for CD[SES-CD]) continued MIRI SC dosing in VIVID-2. Endoscopic non-responders received reinduction with MIRI IV (3x Q4W) at the start of VIVID-2 followed by SC dosing (IV-SC) as described. Pts having an Urgency Numeric Rating Scale (UNRS) ≥3 at baseline were included in these analyses. BU remission was defined as an UNRS score of ≤2. Associations between BU remission with clinical remission by Crohn’s Disease Activity Index (CDAI), endoscopic remission, Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue, and Inflammatory Bowel Disease Questionnaire (IBDQ) remission, were assessed at W104, within the VIVID-1 W52 endoscopic response subgroups and the MIRI total group.
Results: Over 50% of the MIRI total group, 56% MIRI W52 endoscopic responders, and 43% MIRI W52 endoscopic non-responders achieved W104 BU remission. In the MIRI total group, a significantly higher percentage of pts achieved clinical remission, endoscopic remission and IBDQ remission at W104 in the BU remission group vs those without BU remission. The improvement in fatigue by FACIT-Fatigue score was significantly more in the group with BU remission vs those without BU remission (p< 0.0001). In both MIRI W52 endoscopic responders and non-responders, pts who achieved W104 BU remission, vs who did not, achieved significantly higher rates of clinical remission, greater improvements in FACIT-Fatigue, and higher rates of quality of life improvement measured by IBDQ remission (Table).
Discussion: Over 50% pts with CD achieved BU remission at 2 years of MIRI treatment. Achieving BU remission corresponded with improvements to outcomes important to pts with CD, underscoring the importance of long-term management of BU in improving overall pt outcomes.

Figure: Association Between Bowel Urgency Remission and Clinical, Endoscopic, and Patient-reported Outcomes at W104
Disclosures:
Stephan Vavricka: Abbott – Consultant, Grant/Research Support, Speakers Bureau. Alfasigma – Consultant, Grant/Research Support, Speakers Bureau. Amgen – Consultant, Grant/Research Support, Speakers Bureau. Arenapharm – Consultant, Grant/Research Support, Speakers Bureau. BMS – Consultant, Grant/Research Support, Speakers Bureau. Falk Pharma GmbH – Consultant, Grant/Research Support, Speakers Bureau. Ferring Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Gilead – Consultant, Grant/Research Support, Speakers Bureau. iQuone – Consultant, Grant/Research Support, Speakers Bureau. Janssen – Consultant, Grant/Research Support, Speakers Bureau. MSD – Consultant, Grant/Research Support, Speakers Bureau. Permamed – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Sanofi-Aventis – Consultant, Grant/Research Support, Speakers Bureau. Schwabe Pharma – Consultant, Grant/Research Support, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Tillotts – Consultant, Grant/Research Support, Speakers Bureau. UCB – Consultant, Grant/Research Support, Speakers Bureau. Vifor – Consultant, Grant/Research Support, Speakers Bureau.
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Jianmin Wu: Eli Lilly and Company – Employee, Stock Options.
Guanglei Yu: Eli Lilly and Company – Employee, Stock Options.
Aisha Vadhariya: Eli Lilly and Company – Employee, Stock Options.
Richard Moses: Eli Lilly and Company – Employee, Stock Options.
Rebecca Hozak: Eli Lilly and Company – Employee, Stock Options.
Geert D’Haens: Abbvie – Advisor or Review Panel Member, Speakers Bureau. Abivax – Advisor or Review Panel Member. Agomab – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. AMT – Consultant. Anaptys Bio – Advisor or Review Panel Member. AstraZeneca – Consultant. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member. Eli Lilly – Advisor or Review Panel Member. Exeliom – Consultant. Galapagos – Advisor or Review Panel Member. Glaxo Smith Kline – Advisor or Review Panel Member. Gossamerbio – Consultant. Immunic – Consultant. Index Pharmaceuticals – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member. Kaleido – Consultant. Merck – Advisor or Review Panel Member. Origo – Consultant. Pfizer – Consultant, Speakers Bureau. Polpharm – Advisor or Review Panel Member. Procise Diagnostics – Consultant. Progenity – Consultant. Prometheus Biosciences – Advisor or Review Panel Member. Protagonist Therapeutics – Consultant. Sanofi – Advisor or Review Panel Member. Seres Health – Advisory Committee/Board Member. Takeda – Advisor or Review Panel Member. Tillotts – Advisor or Review Panel Member.
Simon Travis: AbbVie – Grant/Research Support, Honoraria, Travel expenses. Alimentiv – Independent Contractor. Bioclinica – Consultant. Bristol Myers Squibb – Consultant. Cosmo – Consultant. Dova Health Intelligence – Consultant, Stock Options. ECCO Health Care – Grant/Research Support. Eli Lilly and Company – Consultant, Grant/Research Support, Honoraria. Equillium – Consultant. Ferring – Consultant, Honoraria, Travel expenses. Genentech – Consultant. GlaxoSmithKline – Consultant. Immunocore – Consultant. Immunometabolism – Consultant. Janssen – Consultant. Mestag – Consultant. MSD – Consultant. Norman Collisson Foundation – Grant/Research Support. Novartis – Consultant. Pfizer – Consultant, Grant/Research Support, Travel expenses. Protagonist – Consultant. Roche – Consultant. Satisfai – Consultant. Sorriso – Consultant. Spyre – Consultant. Takeda – Consultant, Honoraria, Travel expenses. The Helmsley Trust – Grant/Research Support. the International Organization for the Study of Inflammatory Bowel Diseases – Grant/Research Support. UCB – Consultant, Grant/Research Support. UK–India Education and Research Initiative – Grant/Research Support. Vifor – Consultant, Grant/Research Support.
Alissa Walsh: Abbvie – Consultant, Speakers Bureau. Bristol-Myers Squibb – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau.
Stephan Vavricka, MD1, David T. Rubin, MD2, Jianmin Wu, 3, Guanglei Yu, PhD4, Aisha Vadhariya, 3, Richard E.. Moses, DO, JD3, Rebecca Hozak, 3, Geert R. D’Haens, MD, PhD5, Simon Travis, 6, Alissa Walsh, MBBS, PhD6. P5479 - Mirikizumab Long-Term Impact on Bowel Urgency in Crohn’s Disease and Its Association With Clinical and Other Efficacy Outcomes: Results From the VIVID-2 Open-Label Extension Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
