Tuesday Poster Session
Category: IBD
P5484 - Guselkumab Is Efficacious and Safe for Moderately-to-Severe Active Ulcerative Colitis: Real-World Data From a Large Tertiary Center
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
Has Audio
- AS
Asher Shafrir, MD
University of Chicago Medicine
Chicago, IL
Presenting Author(s)
Asher Shafrir, MD1, Alex J. Mathew, MBE2, Jessica Tanouye, 2, Alexandra R.. Hannett, 3, Russell Yanofsky, MD2, David Choi, PharmD4, Russell D. Cohen, MD1, David T. Rubin, MD5
1University of Chicago Medicine, Chicago, IL; 2University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 3University of Chicago Medical Center, Chicago, IL; 4University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 5University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL
Introduction: Guselkumab (GUS) is an IgG1 inhibitor that binds to and inhibits the p19 subunit of interleukin-23, a recognized cytokine involved in the inflammatory processes of inflammatory bowel disease (IBD). GUS has been shown to effectively induce and maintain remission in patients with moderately to severely active ulcerative colitis (UC). We present real-world efficacy and safety of GUS in UC in a large tertiary IBD center.
Methods: All patients prescribed GUS at our center were prospectively monitored. Patients were contacted at weeks 0, 2, 4, 8, and 12. Data regarding disease activity (using the Simple Clinical Colitis Index (SCCAI)), steroid usage, and adverse events were collected, and blood tests were also collected at weeks 0, 4,8, and 12. Data on patients observed up to at least week 8 were collected and analyzed. All patients were evaluated for safety outcomes, and patients with UC were assessed for efficacy
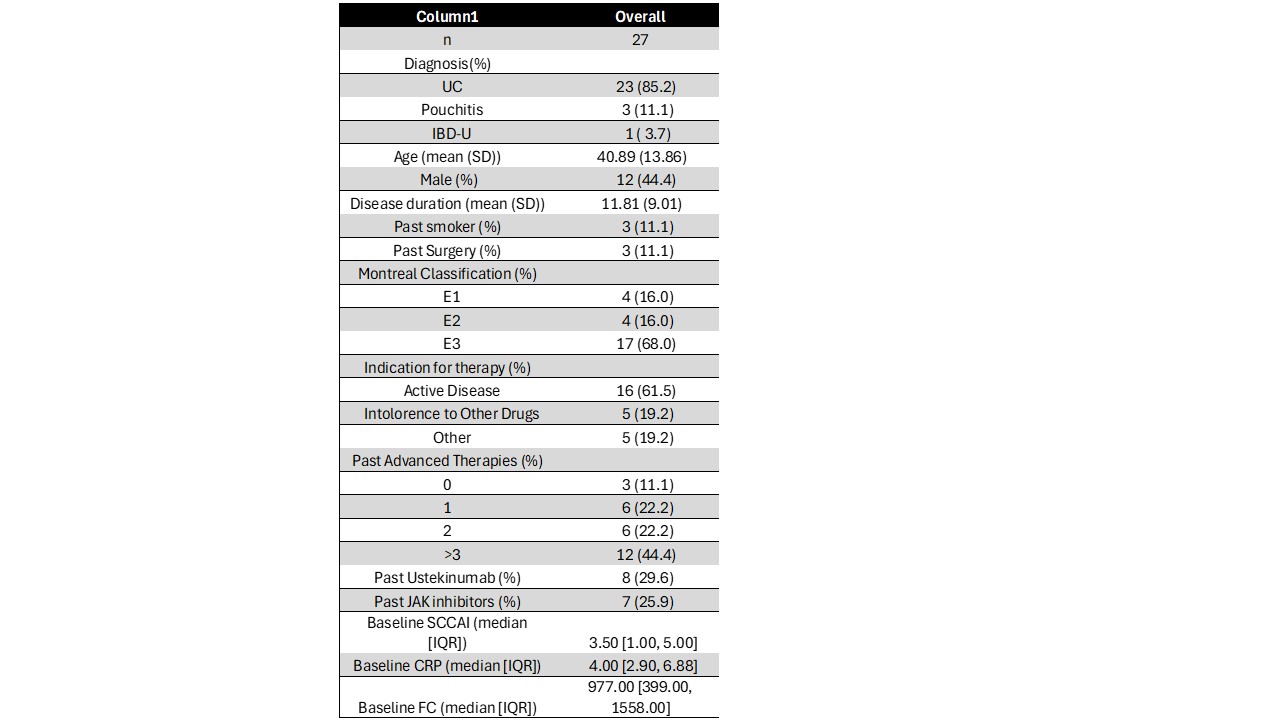
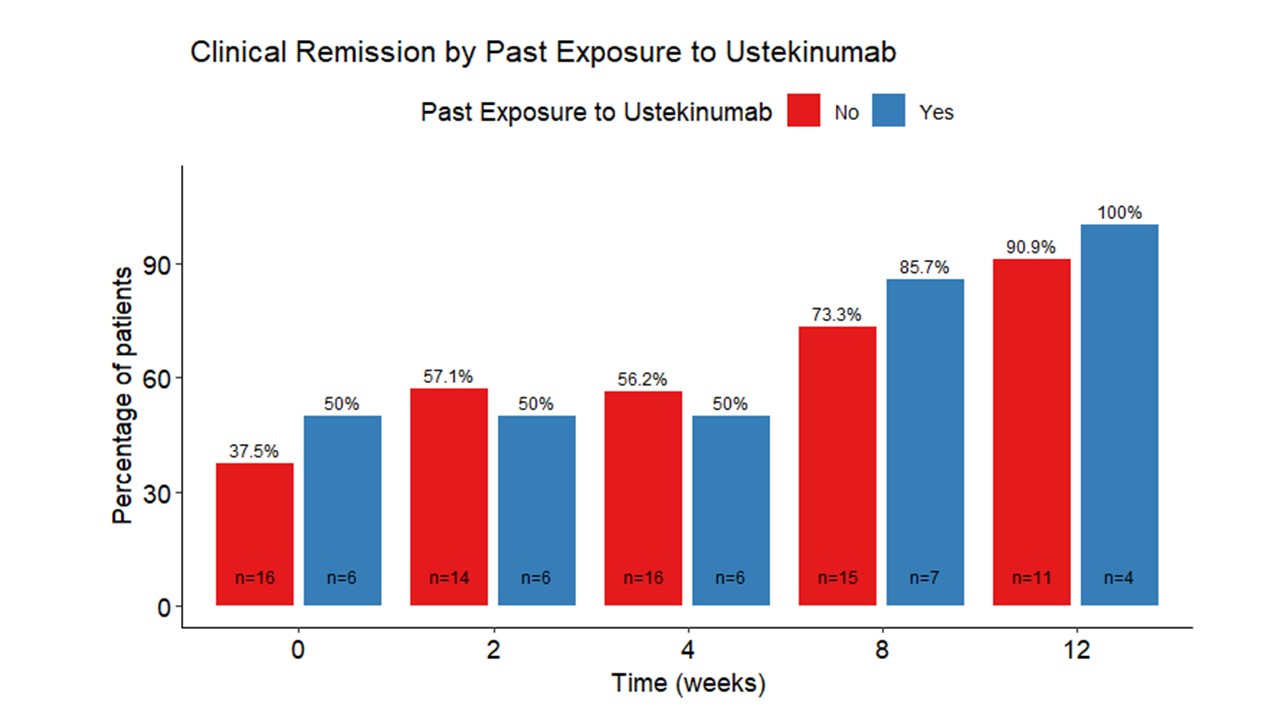
Results: There were 27 patients in the cohort at the time of this analysis (UC: 23, pouchitis: 3, IBD-U: 1; mean age 40.9±13.9, 55.6% female. (Table) At week 8, a total of 26 (96%) patients continued using GUS. Among the 19 patients who reached week 12, 16(84%) maintained their regimen of GUS. Median SCCAI score decreased from 3.5 (interquartile range [IQR] 1-5) at week 0 to 1 (IQR 0-2) at week 8 and 0 at week 12 (IQR 0-1). At week 0, 38.1% were in clinical remission (SCCAI ≤ 2). By week 8, 75% of patients were in clinical remission, and 92.9% were in remission at week 12.Remission rates were similar in patients who were ustekinumab (UST) naïve and exposed. (Figure 1) At the commencement of treatment, 36.4% of the patients received steroid therapy. This proportion decreased to 12% by week 8 and 12.5% by week 12. Steroid-free clinical remission was 24% at week 0, 52.2% at week 8, and 66.7% at week 12. In univariate analysis, age, sex, and prior therapy involving TNF inhibitors or ustekinumab were not associated with steroid-free remission at weeks 8 or 12. Thirty percent had undetectable CRP levels at baseline, compared to 52% at week 8. Median fecal calprotectin dropped from 1089 at baseline to 299 at week 8. There were no significant treatment-related adverse events.
Discussion: In the first real-world analysis of GUS in patients with moderately to severely active UC, GUS demonstrated significant effectiveness and safety, even among prior UST-exposed patients.
Disclosures:
Asher Shafrir, MD1, Alex J. Mathew, MBE2, Jessica Tanouye, 2, Alexandra R.. Hannett, 3, Russell Yanofsky, MD2, David Choi, PharmD4, Russell D. Cohen, MD1, David T. Rubin, MD5. P5484 - Guselkumab Is Efficacious and Safe for Moderately-to-Severe Active Ulcerative Colitis: Real-World Data From a Large Tertiary Center, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Chicago Medicine, Chicago, IL; 2University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 3University of Chicago Medical Center, Chicago, IL; 4University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 5University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL
Introduction: Guselkumab (GUS) is an IgG1 inhibitor that binds to and inhibits the p19 subunit of interleukin-23, a recognized cytokine involved in the inflammatory processes of inflammatory bowel disease (IBD). GUS has been shown to effectively induce and maintain remission in patients with moderately to severely active ulcerative colitis (UC). We present real-world efficacy and safety of GUS in UC in a large tertiary IBD center.
Methods: All patients prescribed GUS at our center were prospectively monitored. Patients were contacted at weeks 0, 2, 4, 8, and 12. Data regarding disease activity (using the Simple Clinical Colitis Index (SCCAI)), steroid usage, and adverse events were collected, and blood tests were also collected at weeks 0, 4,8, and 12. Data on patients observed up to at least week 8 were collected and analyzed. All patients were evaluated for safety outcomes, and patients with UC were assessed for efficacy
Results: There were 27 patients in the cohort at the time of this analysis (UC: 23, pouchitis: 3, IBD-U: 1; mean age 40.9±13.9, 55.6% female. (Table) At week 8, a total of 26 (96%) patients continued using GUS. Among the 19 patients who reached week 12, 16(84%) maintained their regimen of GUS. Median SCCAI score decreased from 3.5 (interquartile range [IQR] 1-5) at week 0 to 1 (IQR 0-2) at week 8 and 0 at week 12 (IQR 0-1). At week 0, 38.1% were in clinical remission (SCCAI ≤ 2). By week 8, 75% of patients were in clinical remission, and 92.9% were in remission at week 12.Remission rates were similar in patients who were ustekinumab (UST) naïve and exposed. (Figure 1) At the commencement of treatment, 36.4% of the patients received steroid therapy. This proportion decreased to 12% by week 8 and 12.5% by week 12. Steroid-free clinical remission was 24% at week 0, 52.2% at week 8, and 66.7% at week 12. In univariate analysis, age, sex, and prior therapy involving TNF inhibitors or ustekinumab were not associated with steroid-free remission at weeks 8 or 12. Thirty percent had undetectable CRP levels at baseline, compared to 52% at week 8. Median fecal calprotectin dropped from 1089 at baseline to 299 at week 8. There were no significant treatment-related adverse events.
Discussion: In the first real-world analysis of GUS in patients with moderately to severely active UC, GUS demonstrated significant effectiveness and safety, even among prior UST-exposed patients.

Figure: Baric Characteristics of Patients Receiving Guselkumab

Figure: Remission Rates in Patients Who Were Ustekinumab Naïve and Exposed.
Disclosures:
Asher Shafrir indicated no relevant financial relationships.
Alex Mathew indicated no relevant financial relationships.
Jessica Tanouye indicated no relevant financial relationships.
Alexandra Hannett indicated no relevant financial relationships.
Russell Yanofsky indicated no relevant financial relationships.
David Choi: Abbvie – Advisory Committee/Board Member, Speakers Bureau. Boehringer – Advisory Committee/Board Member. Bristol Myers Squibb – Advisory Committee/Board Member. Eli Lilly – Advisory Committee/Board Member, Speakers Bureau. Johnson and Johnso – Advisory Committee/Board Member, Speakers Bureau. Pfizer – Advisory Committee/Board Member.
Russell Cohen: Abbvie – Consultant, Speakers Bureau. Bausch Health – Consultant. BMS – Consultant. Eli lilly – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. Johnson & Johnson – Consultant. Pfizer – Consultant. Takeda – Consultant.
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Asher Shafrir, MD1, Alex J. Mathew, MBE2, Jessica Tanouye, 2, Alexandra R.. Hannett, 3, Russell Yanofsky, MD2, David Choi, PharmD4, Russell D. Cohen, MD1, David T. Rubin, MD5. P5484 - Guselkumab Is Efficacious and Safe for Moderately-to-Severe Active Ulcerative Colitis: Real-World Data From a Large Tertiary Center, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
