Tuesday Poster Session
Category: Infections and Microbiome
P5575 - Effectiveness of Colonoscopic and Capsule Fecal Microbiota Transplantation for Prevention of Recurrent Clostridioides difficile Infection: A Prospective Cohort Study
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Has Audio
- DB
Dina Belhasan, MD
University of Minnesota
Minneapolis, MN
Presenting Author(s)
Award: ACG Presidential Poster Award
Dina Belhasan, MD1, Nataliia Kuchma, MD, PhD2, Monika Fischer, MD, MS3, Jessica R.. Allegretti, MD, MPH4, Colleen R. Kelly, MD5, Alexander Khoruts, MD2, Byron Vaughn, MD, MS2
1University of Minnesota, Minneapolis, MN; 2University of Minnesota Medical Center, Minneapolis, MN; 3Indiana University-Purdue University Indianapolis, Indianapolis, IN; 4Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 5Brigham and Women's Hospital, Providence, RI
Introduction: Fecal microbiota transplant (FMT) is effective for preventing recurrent Clostridioides difficile infection (rCDI). Previous comparisons between oral and colonoscopic FMT have been limited by small sample sizes. In an earlier version of our cohort (n=269), no significant difference in efficacy was observed. This study reassessed outcomes in a larger cohort.
Methods: We analyzed a prospective cohort of patients with recurrent CDI treated with FMT between 7/1/19-11/29/23 across six centers. Patients received liquid or oral capsule FMT formulations at the discretion of treating physicians. Follow-up occurred at 1, 2, 6, and 12 months. Only first CDI relapses were counted as recurrences. Analyses used Fisher exact tests (Rv4.4.3).
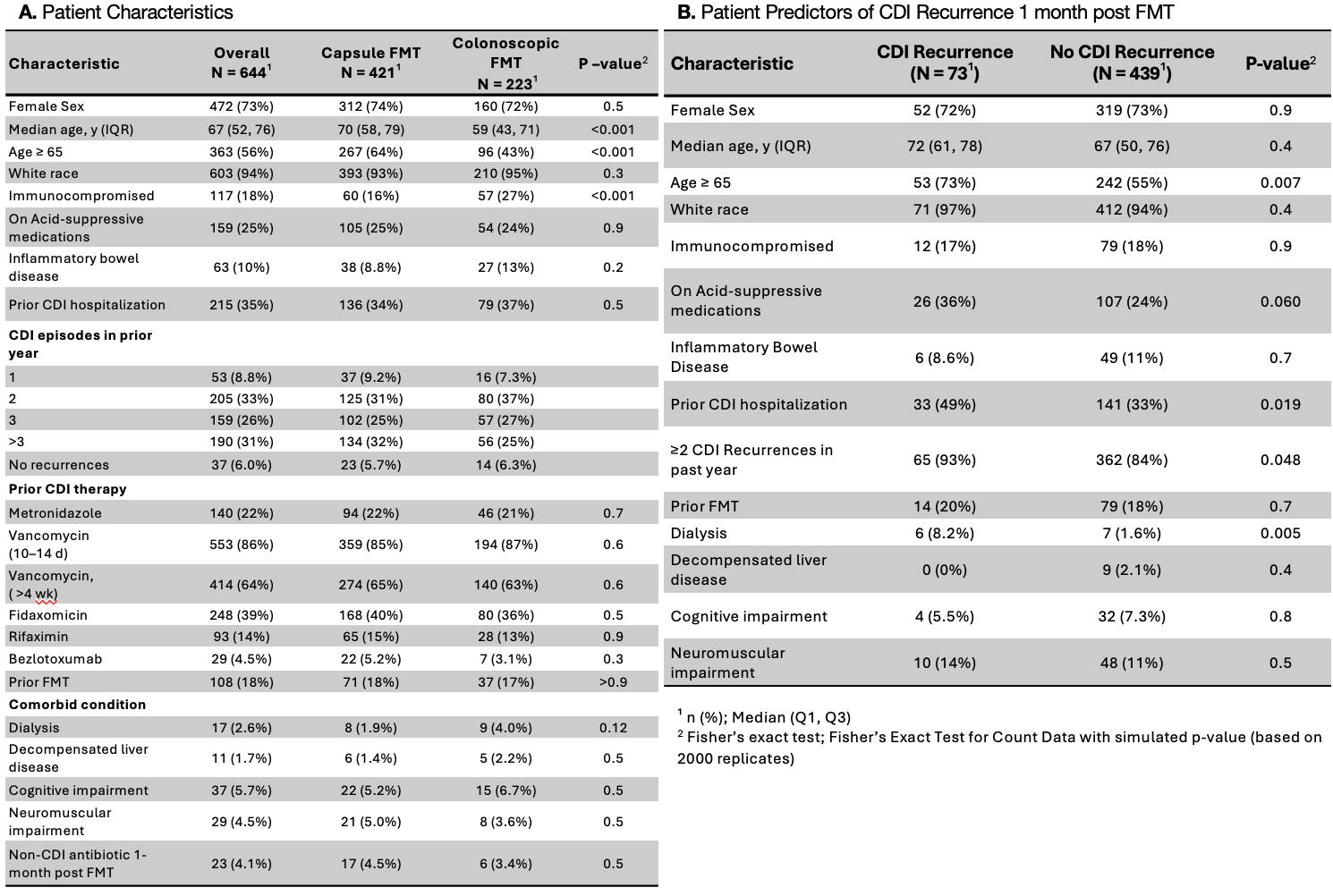
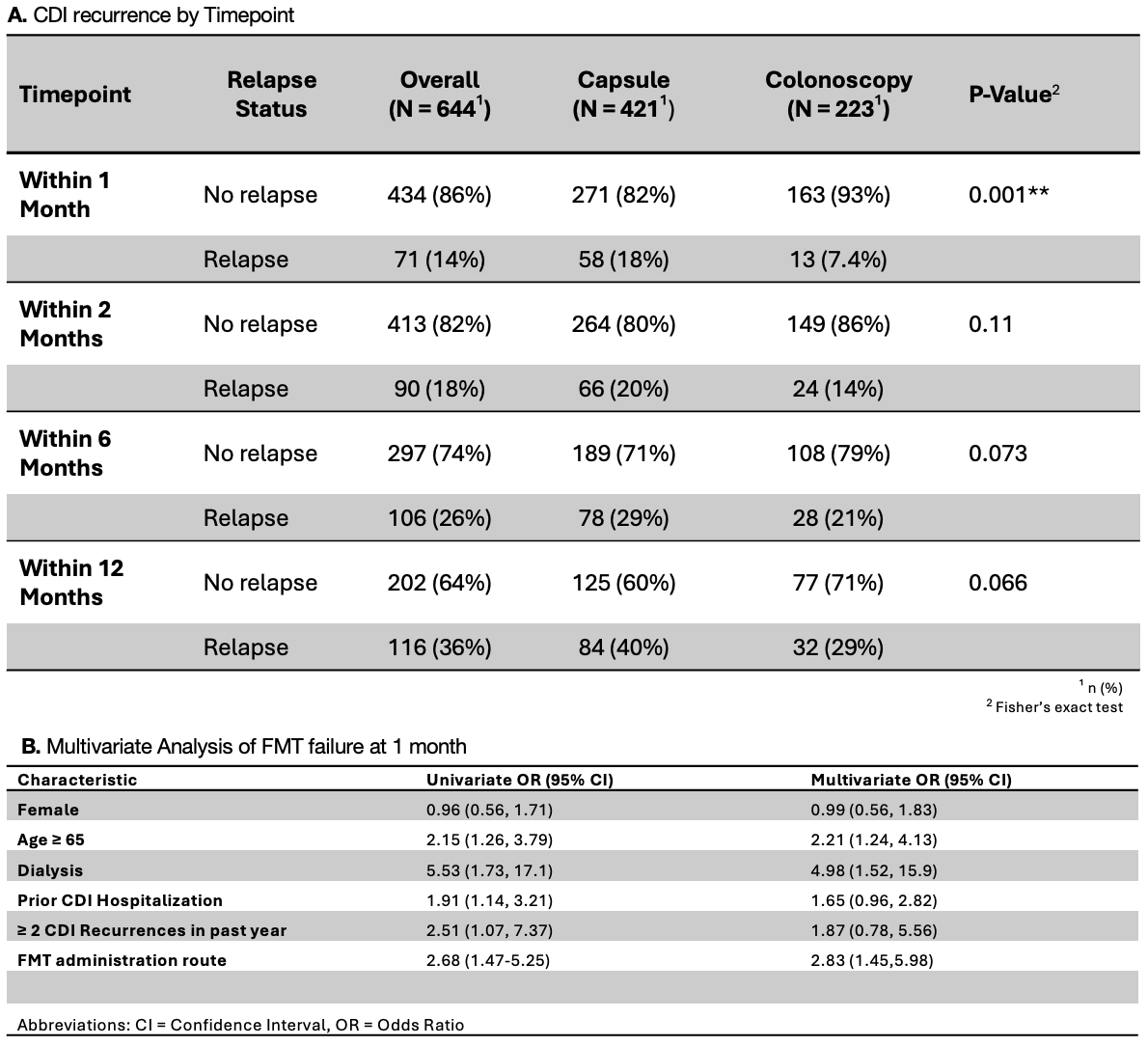
Results: A total of 644 individuals received FMT (capsule=421, colonoscopy=233). The crude 1-month CDI recurrence rate was higher with capsule compared to colonoscopy FMT (18% vs.7.4%, p = 0.001), with no significant differences at later time points. Capsule recipients were older (median age 70 vs. 59, p < 0.001), while colonoscopic recipients were more often immunocompromised (27% vs.15%, p < 0.001); IBD status and non-CDI antibiotic use post-FMT were similar across groups. Age ≥65, ≥2 past CDI recurrences, prior CDI hospitalizations, and dialysis were independent predictors of CDI recurrence. After adjusting for confounders, capsule FMT was associated with increased rCDI at 1-month (OR: 2.83, 95% CI: 1.45–5.98, p = 0.004). Among patients ≥65 years, capsule FMT was associated with a 2.7-fold increased rCDI risk compared to colonoscopy (95% CI: 1.2–6.4, p = 0.02), whereas no significant difference was observed in patients < 65 years (OR: 1.9, 95% CI:0.7–5.3, p = 0.2).
Discussion: Capsule FMT was less effective than colonoscopic FMT at preventing rCDI at 1-month, particularly in those aged ≥ 65. Although recurrence rates equalized beyond 1-month, most relapses occurred within this initial period. Age differences raise the possibility of residual confounding, though differential microbial engraftment by administration route may also contribute; notably, lack of bowel prep in capsule recipients may impair engraftment. Recurrences beyond 30 days may be more influenced by antibiotic exposure than FMT route. In conclusion, FMT efficacy may vary by route, with capsule FMT potentially less effective in older adults.
Disclosures:
Dina Belhasan, MD1, Nataliia Kuchma, MD, PhD2, Monika Fischer, MD, MS3, Jessica R.. Allegretti, MD, MPH4, Colleen R. Kelly, MD5, Alexander Khoruts, MD2, Byron Vaughn, MD, MS2. P5575 - Effectiveness of Colonoscopic and Capsule Fecal Microbiota Transplantation for Prevention of Recurrent <i>Clostridioides difficile</i> Infection: A Prospective Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Dina Belhasan, MD1, Nataliia Kuchma, MD, PhD2, Monika Fischer, MD, MS3, Jessica R.. Allegretti, MD, MPH4, Colleen R. Kelly, MD5, Alexander Khoruts, MD2, Byron Vaughn, MD, MS2
1University of Minnesota, Minneapolis, MN; 2University of Minnesota Medical Center, Minneapolis, MN; 3Indiana University-Purdue University Indianapolis, Indianapolis, IN; 4Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 5Brigham and Women's Hospital, Providence, RI
Introduction: Fecal microbiota transplant (FMT) is effective for preventing recurrent Clostridioides difficile infection (rCDI). Previous comparisons between oral and colonoscopic FMT have been limited by small sample sizes. In an earlier version of our cohort (n=269), no significant difference in efficacy was observed. This study reassessed outcomes in a larger cohort.
Methods: We analyzed a prospective cohort of patients with recurrent CDI treated with FMT between 7/1/19-11/29/23 across six centers. Patients received liquid or oral capsule FMT formulations at the discretion of treating physicians. Follow-up occurred at 1, 2, 6, and 12 months. Only first CDI relapses were counted as recurrences. Analyses used Fisher exact tests (Rv4.4.3).
Results: A total of 644 individuals received FMT (capsule=421, colonoscopy=233). The crude 1-month CDI recurrence rate was higher with capsule compared to colonoscopy FMT (18% vs.7.4%, p = 0.001), with no significant differences at later time points. Capsule recipients were older (median age 70 vs. 59, p < 0.001), while colonoscopic recipients were more often immunocompromised (27% vs.15%, p < 0.001); IBD status and non-CDI antibiotic use post-FMT were similar across groups. Age ≥65, ≥2 past CDI recurrences, prior CDI hospitalizations, and dialysis were independent predictors of CDI recurrence. After adjusting for confounders, capsule FMT was associated with increased rCDI at 1-month (OR: 2.83, 95% CI: 1.45–5.98, p = 0.004). Among patients ≥65 years, capsule FMT was associated with a 2.7-fold increased rCDI risk compared to colonoscopy (95% CI: 1.2–6.4, p = 0.02), whereas no significant difference was observed in patients < 65 years (OR: 1.9, 95% CI:0.7–5.3, p = 0.2).
Discussion: Capsule FMT was less effective than colonoscopic FMT at preventing rCDI at 1-month, particularly in those aged ≥ 65. Although recurrence rates equalized beyond 1-month, most relapses occurred within this initial period. Age differences raise the possibility of residual confounding, though differential microbial engraftment by administration route may also contribute; notably, lack of bowel prep in capsule recipients may impair engraftment. Recurrences beyond 30 days may be more influenced by antibiotic exposure than FMT route. In conclusion, FMT efficacy may vary by route, with capsule FMT potentially less effective in older adults.

Figure: Figure 1:
A. Patient Characteristics
B. Patient Predictors of CDI Recurrence 1-month post FMT
A. Patient Characteristics
B. Patient Predictors of CDI Recurrence 1-month post FMT

Figure: Figure 2:
A. CDI Recurrence by Timepoint
B. Multivariate Analysis of FMT failure at 1 month
A. CDI Recurrence by Timepoint
B. Multivariate Analysis of FMT failure at 1 month
Disclosures:
Dina Belhasan indicated no relevant financial relationships.
Nataliia Kuchma indicated no relevant financial relationships.
Monika Fischer: abbvie – Advisory Committee/Board Member. Criscot – DSMB chair. Eli Lilly – Advisor or Review Panel Member. Ferring – Advisory Committee/Board Member. Johnson and Johnson – Advisor or Review Panel Member, Speakers Bureau. seres – Advisor or Review Panel Member, Advisory Committee/Board Member.
Jessica Allegretti: Abbvie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker, Speakers Bureau. Adiso – Consultant. Bristol Myer Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Finch – Consultant. Genentech – Advisory Committee/Board Member, Consultant. GlaxoSmithKline – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Iterative Scopes – Consultant. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speaker. Merck – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Roivant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Roivant Adiso – Consultant. Seres Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Shattuck Labs – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. TRXBio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Vedanta – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant.
Colleen Kelly: Eli Lilly and Company – Advisory Committee/Board Member.
Alexander Khoruts indicated no relevant financial relationships.
Byron Vaughn: Diasorin – Grant/Research Support. Health Delegates – Advisory Committee/Board Member. Kate Farms – Grant/Research Support. Takeda – Grant/Research Support.
Dina Belhasan, MD1, Nataliia Kuchma, MD, PhD2, Monika Fischer, MD, MS3, Jessica R.. Allegretti, MD, MPH4, Colleen R. Kelly, MD5, Alexander Khoruts, MD2, Byron Vaughn, MD, MS2. P5575 - Effectiveness of Colonoscopic and Capsule Fecal Microbiota Transplantation for Prevention of Recurrent <i>Clostridioides difficile</i> Infection: A Prospective Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

