Tuesday Poster Session
Category: Infections and Microbiome
P5576 - Antibiotic Exposure Is a Short- and Long-Term Risk for Recurrent Clostridioides difficile Infection Following Fecal Microbiota Transplantation
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Has Audio
- DB
Dina Belhasan, MD
University of Minnesota
Minneapolis, MN
Presenting Author(s)
Dina Belhasan, MD1, Nataliia Kuchma, MD, PhD2, Jessica R.. Allegretti, MD, MPH3, Monika Fischer, MD, MS4, Alexander Khoruts, MD2, Colleen R. Kelly, MD5, Byron Vaughn, MD, MS2
1University of Minnesota, Minneapolis, MN; 2University of Minnesota Medical Center, Minneapolis, MN; 3Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 4Indiana University-Purdue University Indianapolis, Indianapolis, IN; 5Brigham and Women's Hospital, Providence, RI
Introduction: Fecal microbiota transplant (FMT) is an effective treatment for recurrent Clostridioides difficile infection (rCDI) after failure of standard therapies. Antibiotic exposure within 2-months post-FMT is a known risk factor for early recurrence. However, the timeline of antibiotics administration later in the post-FMT period and longer-term recurrence remains poorly studied. This study evaluates the association between antibiotic exposure at various intervals within 12 months post-FMT and rCDI.
Methods: Secondary analysis of a prospective cohort study included patients with history of rCDI treated with FMT between 7/1/19-11/29/23 across six centers. Follow-up occurred at 1, 2, 6, and 12 months, recording non-CDI antibiotic use and rCDI at each interval; exact dates were unavailable. The cohort was analyzed by follow-up time with individuals who had complete data for that interval. Following CDI recurrence, a participant was excluded from future timepoints. CDI recurrence was defined as laboratory positive testing (per local practice) and resumption of antibiotics for CDI.
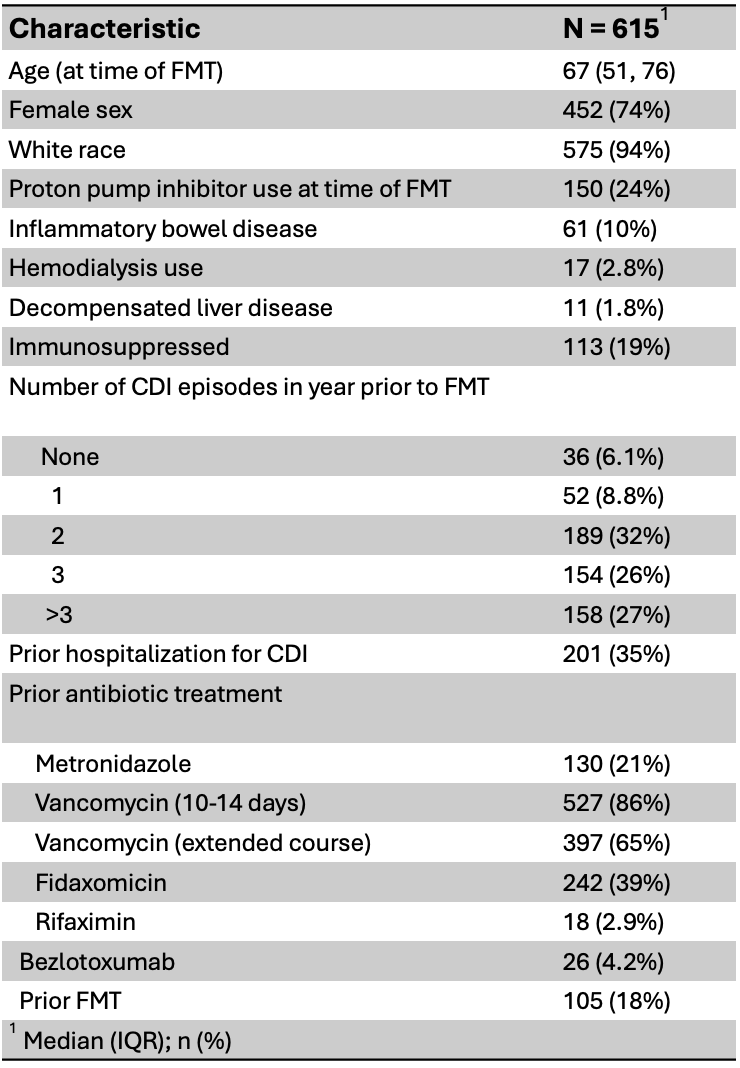
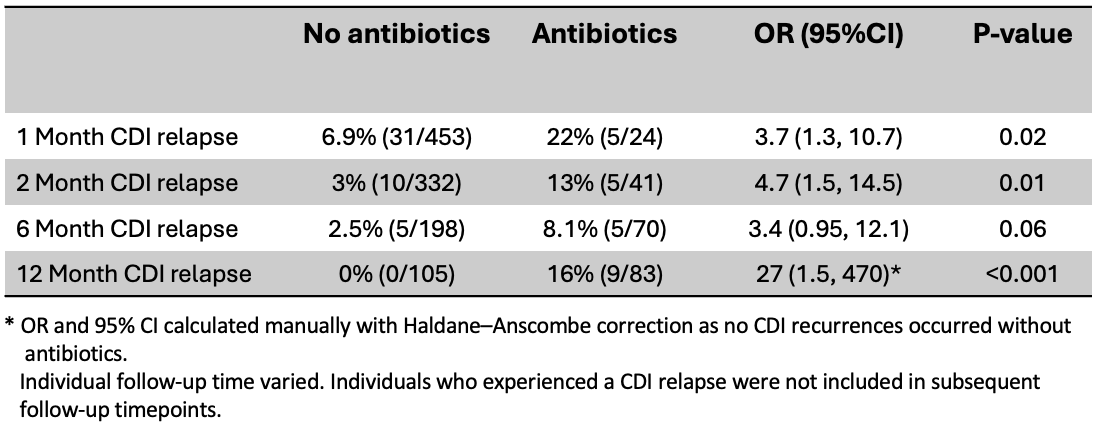
Results: Of 701 patients in the cohort, 615 met the inclusion criteria, though not all individuals had follow-up at each timepoint. Most patients had 3 or more rCDI episodes prior to FMT (Table 1). Non-CDI antibiotic administration was associated with CDI recurrence at 1-, 2-, and 12-months, with a trend towards significance at 6-months (Table 2). Overall, the odds of developing CDI were 3-5 times higher following antibiotic exposure compared to no additional antibiotic exposure. After 1month, the risk of a spontaneous CDI recurrence (i.e., no additional antibiotic exposures) was < 5%. Between 6 and 12 months, all CDI recurrences occurred following one or more courses of non-CDI antibiotics.
Discussion: Antibiotic use post-FMT is a key driver of CDI recurrence. Prior studies have identified non-CDI antibiotic exposure as a risk factor for early recurrence (within 2 months). Our cohort replicates this finding, and also demonstrates that antibiotic exposure continues to be a risk for CDI recurrence for at least 1-year post FMT. Recall bias may be a limitation, as patients with recurrence may more readily remember antibiotic use than those without. Overall, our findings support caution in prescribing non-CDI antibiotics for at least a year following FMT for CDI.
Disclosures:
Dina Belhasan, MD1, Nataliia Kuchma, MD, PhD2, Jessica R.. Allegretti, MD, MPH3, Monika Fischer, MD, MS4, Alexander Khoruts, MD2, Colleen R. Kelly, MD5, Byron Vaughn, MD, MS2. P5576 - Antibiotic Exposure Is a Short- and Long-Term Risk for Recurrent <i>Clostridioides difficile</i> Infection Following Fecal Microbiota Transplantation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Minnesota, Minneapolis, MN; 2University of Minnesota Medical Center, Minneapolis, MN; 3Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 4Indiana University-Purdue University Indianapolis, Indianapolis, IN; 5Brigham and Women's Hospital, Providence, RI
Introduction: Fecal microbiota transplant (FMT) is an effective treatment for recurrent Clostridioides difficile infection (rCDI) after failure of standard therapies. Antibiotic exposure within 2-months post-FMT is a known risk factor for early recurrence. However, the timeline of antibiotics administration later in the post-FMT period and longer-term recurrence remains poorly studied. This study evaluates the association between antibiotic exposure at various intervals within 12 months post-FMT and rCDI.
Methods: Secondary analysis of a prospective cohort study included patients with history of rCDI treated with FMT between 7/1/19-11/29/23 across six centers. Follow-up occurred at 1, 2, 6, and 12 months, recording non-CDI antibiotic use and rCDI at each interval; exact dates were unavailable. The cohort was analyzed by follow-up time with individuals who had complete data for that interval. Following CDI recurrence, a participant was excluded from future timepoints. CDI recurrence was defined as laboratory positive testing (per local practice) and resumption of antibiotics for CDI.
Results: Of 701 patients in the cohort, 615 met the inclusion criteria, though not all individuals had follow-up at each timepoint. Most patients had 3 or more rCDI episodes prior to FMT (Table 1). Non-CDI antibiotic administration was associated with CDI recurrence at 1-, 2-, and 12-months, with a trend towards significance at 6-months (Table 2). Overall, the odds of developing CDI were 3-5 times higher following antibiotic exposure compared to no additional antibiotic exposure. After 1month, the risk of a spontaneous CDI recurrence (i.e., no additional antibiotic exposures) was < 5%. Between 6 and 12 months, all CDI recurrences occurred following one or more courses of non-CDI antibiotics.
Discussion: Antibiotic use post-FMT is a key driver of CDI recurrence. Prior studies have identified non-CDI antibiotic exposure as a risk factor for early recurrence (within 2 months). Our cohort replicates this finding, and also demonstrates that antibiotic exposure continues to be a risk for CDI recurrence for at least 1-year post FMT. Recall bias may be a limitation, as patients with recurrence may more readily remember antibiotic use than those without. Overall, our findings support caution in prescribing non-CDI antibiotics for at least a year following FMT for CDI.

Figure: Table 1: Patient characteristics

Figure: Table 2: Proportion of CDI recurrence at each follow-up time with and without additional non-CDI
antibiotic exposure.
antibiotic exposure.
Disclosures:
Dina Belhasan indicated no relevant financial relationships.
Nataliia Kuchma indicated no relevant financial relationships.
Jessica Allegretti: Abbvie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker, Speakers Bureau. Adiso – Consultant. Bristol Myer Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Finch – Consultant. Genentech – Advisory Committee/Board Member, Consultant. GlaxoSmithKline – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Iterative Scopes – Consultant. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speaker. Merck – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Roivant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Roivant Adiso – Consultant. Seres Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Shattuck Labs – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. TRXBio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Vedanta – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant.
Monika Fischer: abbvie – Advisory Committee/Board Member. Criscot – DSMB chair. Eli Lilly – Advisor or Review Panel Member. Ferring – Advisory Committee/Board Member. Johnson and Johnson – Advisor or Review Panel Member, Speakers Bureau. seres – Advisor or Review Panel Member, Advisory Committee/Board Member.
Alexander Khoruts indicated no relevant financial relationships.
Colleen Kelly: Eli Lilly and Company – Advisory Committee/Board Member.
Byron Vaughn: Diasorin – Grant/Research Support. Health Delegates – Advisory Committee/Board Member. Kate Farms – Grant/Research Support. Takeda – Grant/Research Support.
Dina Belhasan, MD1, Nataliia Kuchma, MD, PhD2, Jessica R.. Allegretti, MD, MPH3, Monika Fischer, MD, MS4, Alexander Khoruts, MD2, Colleen R. Kelly, MD5, Byron Vaughn, MD, MS2. P5576 - Antibiotic Exposure Is a Short- and Long-Term Risk for Recurrent <i>Clostridioides difficile</i> Infection Following Fecal Microbiota Transplantation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
