Monday Poster Session
Category: Liver
P3860 - A Case Report: Drug-Induced Liver Injury from Prolonged Topical Application of Noni (Morinda citrifolia)
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Has Audio

Diego J. Duran Baez, MD
Mount Sinai South Nassau,Icahn School of Medicine at Mount Sinai
Uniondale, NY
Presenting Author(s)
Charles Kim, PhD1, Diego Baez, MD1, Lenura Ziyadin, DO1, Samuel Huang, MD, MS1, Jonathan Vincent Reyes, MD2, Mahnoor Khan, MD3, Keith Brennan, MD1
1Mount Sinai South Nassau, Oceanside, NY; 2Mount Sinai South Nassau,Icahn School of Medicine at Mount Sinai, Oceanside, NY; 3Columbia University Irving Medical Center, New York, NY
Introduction: Noni (Morinda citrifolia) is a plant-based supplement that has antioxidant, anti-inflammatory and immunomodulating effects. It can be found in many formulations including oral supplements, juice, topical ointments. There have been sparse case reports on the juice and oral supplementations of Noni associated with transaminitis. However, there have been no reported instances of transaminitis linked to the topical use of Noni.
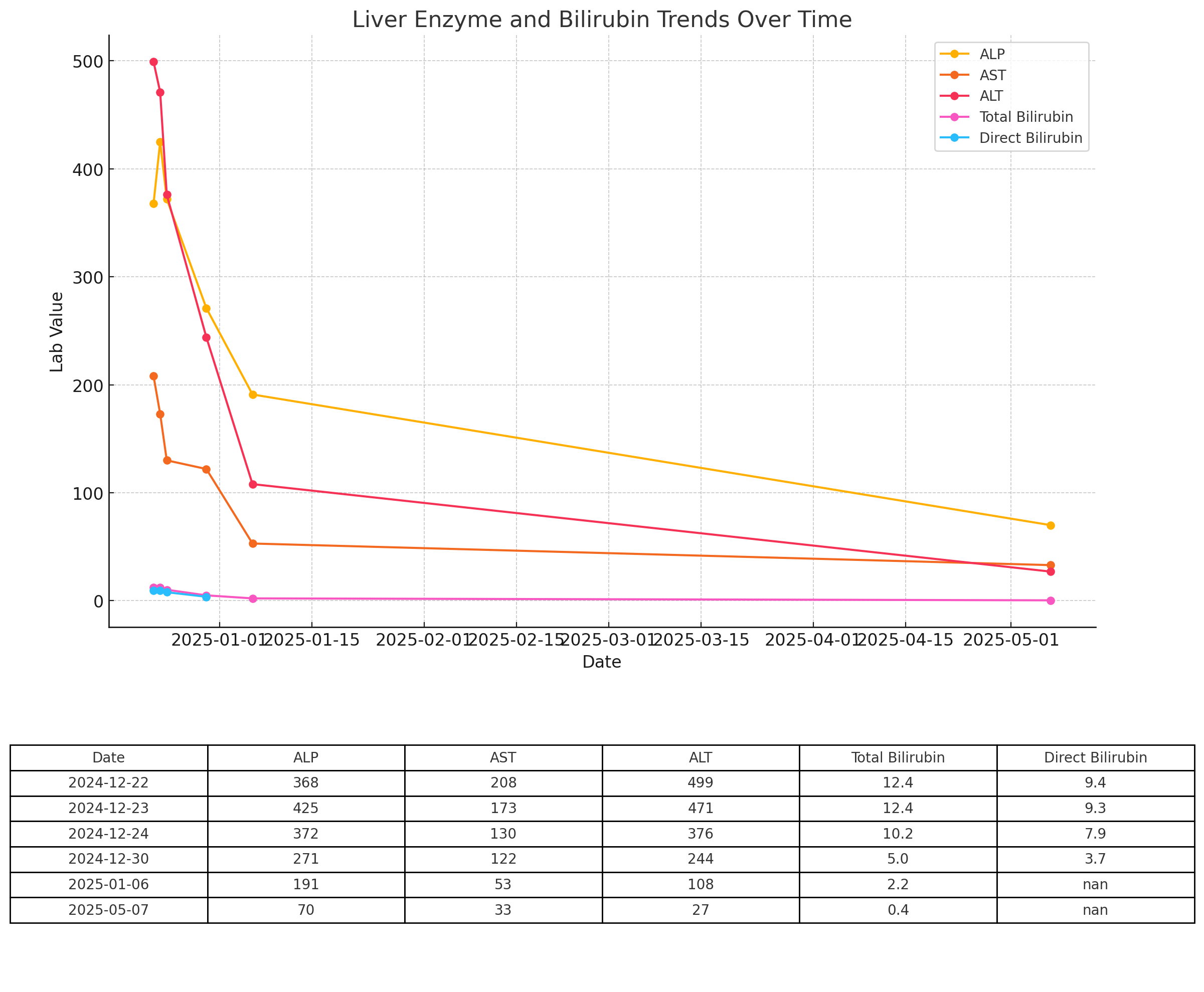
Case Description/Methods: We present a case of a 60-year-old male with a history of hypertension and hypothyroidism presented with jaundice, generalized pruritus, dark yellow urine, and acholic stools persisting for five days. Laboratory findings revealed elevated liver enzymes and a cholestatic pattern of injury. The patient had used a topical Noni (Morinda citrifolia )-based analgesic cream for eight months before discontinuing it three months prior to the onset of symptoms. Extensive diagnostic evaluation excluded viral, autoimmune, and metabolic causes of liver dysfunction, leading to a diagnosis of drug-induced liver injury secondary to the prolonged use of Noni products. Patient was closely monitored outpatient after discontinuation of topical Noni with normalization of the liver function tests within six months.
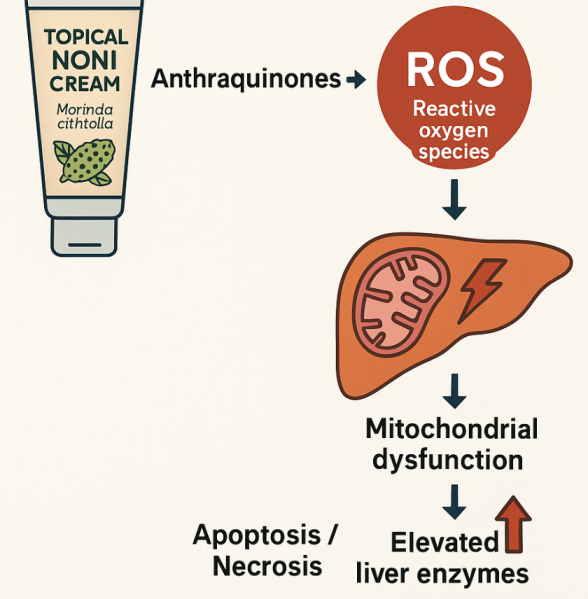
Discussion: The elevation of liver enzymes secondary to the use of topical Noni is exceptionally rare in literature. Prior case reports showed the association through systemic means of absorption, but not through topical use. This case highlights the hepatotoxic potential of Morinda citrifolia, with topical application, and the importance of recognizing herbal products as potential contributors to liver injury. While the true mechanism of hepatotoxicity is not fully understood, one proposed mechanism could be anthraquinones' induced production of reactive oxygen species leading to the disruption of mitochondrial respiration, impairment energy production in hepatocytes that leads to cell apoptosis or necrosis. Further studies are needed to delineate the mechanism of absorption and toxicity for topical noni products.
Disclosures:
Charles Kim, PhD1, Diego Baez, MD1, Lenura Ziyadin, DO1, Samuel Huang, MD, MS1, Jonathan Vincent Reyes, MD2, Mahnoor Khan, MD3, Keith Brennan, MD1. P3860 - A Case Report: Drug-Induced Liver Injury from Prolonged Topical Application of Noni (Morinda citrifolia), ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mount Sinai South Nassau, Oceanside, NY; 2Mount Sinai South Nassau,Icahn School of Medicine at Mount Sinai, Oceanside, NY; 3Columbia University Irving Medical Center, New York, NY
Introduction: Noni (Morinda citrifolia) is a plant-based supplement that has antioxidant, anti-inflammatory and immunomodulating effects. It can be found in many formulations including oral supplements, juice, topical ointments. There have been sparse case reports on the juice and oral supplementations of Noni associated with transaminitis. However, there have been no reported instances of transaminitis linked to the topical use of Noni.
Case Description/Methods: We present a case of a 60-year-old male with a history of hypertension and hypothyroidism presented with jaundice, generalized pruritus, dark yellow urine, and acholic stools persisting for five days. Laboratory findings revealed elevated liver enzymes and a cholestatic pattern of injury. The patient had used a topical Noni (Morinda citrifolia )-based analgesic cream for eight months before discontinuing it three months prior to the onset of symptoms. Extensive diagnostic evaluation excluded viral, autoimmune, and metabolic causes of liver dysfunction, leading to a diagnosis of drug-induced liver injury secondary to the prolonged use of Noni products. Patient was closely monitored outpatient after discontinuation of topical Noni with normalization of the liver function tests within six months.
Discussion: The elevation of liver enzymes secondary to the use of topical Noni is exceptionally rare in literature. Prior case reports showed the association through systemic means of absorption, but not through topical use. This case highlights the hepatotoxic potential of Morinda citrifolia, with topical application, and the importance of recognizing herbal products as potential contributors to liver injury. While the true mechanism of hepatotoxicity is not fully understood, one proposed mechanism could be anthraquinones' induced production of reactive oxygen species leading to the disruption of mitochondrial respiration, impairment energy production in hepatocytes that leads to cell apoptosis or necrosis. Further studies are needed to delineate the mechanism of absorption and toxicity for topical noni products.

Figure: Timeline of resolution of transamanitis in a cholestatic pattern after discontinuation of noni topical.

Figure: Proposed mechanism based on literature for topical noni and liver toxicity.
Disclosures:
Charles Kim indicated no relevant financial relationships.
Diego Baez indicated no relevant financial relationships.
Lenura Ziyadin indicated no relevant financial relationships.
Samuel Huang indicated no relevant financial relationships.
Jonathan Vincent Reyes indicated no relevant financial relationships.
Mahnoor Khan indicated no relevant financial relationships.
Keith Brennan indicated no relevant financial relationships.
Charles Kim, PhD1, Diego Baez, MD1, Lenura Ziyadin, DO1, Samuel Huang, MD, MS1, Jonathan Vincent Reyes, MD2, Mahnoor Khan, MD3, Keith Brennan, MD1. P3860 - A Case Report: Drug-Induced Liver Injury from Prolonged Topical Application of Noni (Morinda citrifolia), ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
