Sunday Poster Session
Category: Colon
P0379 - Uncovering a Clinical Conundrum: Differentiating Capecitabine Toxicity From Immune Checkpoint Inhibitory Colitis

Vineesha Kollipara, DO, MPH
Medical College of Wisconsin
Milwaukee, WI
Presenting Author(s)
1Medical College of Wisconsin, Milwaukee, WI; 2Medical College of Wisconsin, Wauwatosa, WI
Introduction:
Capecitabine is an oral chemotherapy agent used for metastatic colorectal and breast cancer. It is the prodrug of 5-fluorouracil (5-FU). The DPYD gene encodes dihydropyrimidine dehydrogenase, the rate-limiting enzyme in its metabolism1. Patients with DPYD mutations are at risk of 5-FU toxicity upon exposure1. Capecitabine toxicity and ICI colitis produce similar symptoms and endoscopic findings. Herein, we describe a case of acute colitis in the setting of exposure to multiple oncologic therapies with potential for overlapping gastrointestinal side-effects.
Case Description/Methods:
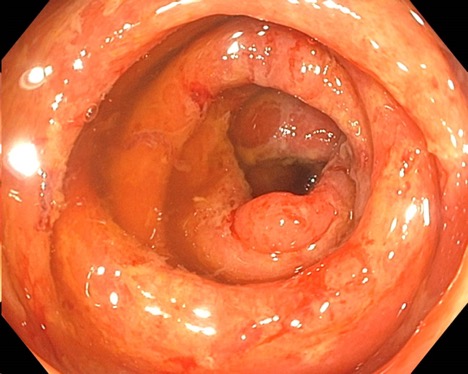
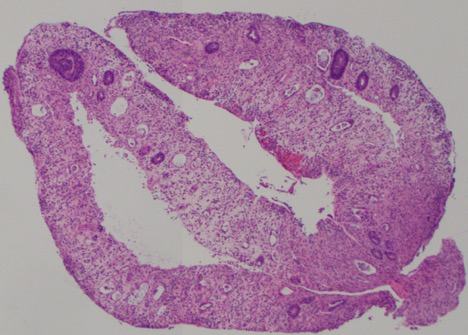
A 68-year-old female with stage IIIA (T3N1M0) breast cancer presented for 10 days of progressive diarrhea. She completed her 8th cycle of pembrolizumab 3 weeks prior and started capecitabine 2 weeks prior. Physical exam noted diffuse abdominal tenderness and fever (102.9°F). Labs noted pancytopenia, hyperbilirubinemia (1.6), elevated CRP (2.34), and negative infectious stool studies. CT abdomen/pelvis noted mild colonic wall thickening. Flexible sigmoidoscopy revealed patchy ulceration, edema, and spontaneous bleeding in the sigmoid colon, with erythema and decreased vascularity in the rectum (Figure 1). Pathology revealed severely active colitis with extensive crypt drop-out and ulceration, favoring capecitabine induced mucosal injury (Figure 2). Subsequent genetic testing identified a DPYD variant designating her an intermediate metabolizer of fluoropyrimidines. Based on the genetic testing and histology, she was diagnosed with a rare side effect of capecitabine induced colitis. She continued her pembrolizumab and was restarted on capecitabine at 50% of her prior dose.
Discussion:
In this case, the histopathology showed crypt atrophy and necrosis- the pervasiveness and severity of which were more characteristic of capecitabine toxicity. Capecitabine toxicity also tends to produce a more neutrophil-predominant inflammatory infiltrate than ICI colitis. Guidelines from the Clinical Pharmacogenetics Implementation Consortium provide recommendations for dosing capecitabine in patients with DPYD mutations.
Identification of capecitabine toxicity based on histopathology led to dose reduction instead of discontinuation, thus preserving the patient’s treatment plan and previously favorable clinical trajectory. This testing should be considered in the setting of dual therapy with a checkpoint inhibitor or in the setting of colitis.
1Meulendijks, et al. The Lancet Oncology. 2015. P1639-1650.
Disclosures:
Vineesha Kollipara, DO, MPH1, Harrison Mooers, MD1, Mahmoud Ali, MD1, Gokulakrishnan Balasubramanian, MD1, Daniel J. Stein, MD2. P0379 - Uncovering a Clinical Conundrum: Differentiating Capecitabine Toxicity From Immune Checkpoint Inhibitory Colitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

