Sunday Poster Session
Category: Colon
P0282 - Association Between ICI and Chronic Colon Mucosal Inflammation: IBD and Microscopic Colitis Risk


Danah Al-Deiri, MD
Cleveland Clinic Foundation
Cleveland, OH
Presenting Author(s)
1Cleveland Clinic Foundation, Cleveland, OH; 2Cleveland Clinic, Cleveland, OH; 3University Hospitals Cleveland Medical Center, Case Western Reserve University, Cleveland, OH
Introduction:
Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment but are associated with immune-related adverse events including gastrointestinal toxicity. The risk of de novo inflammatory bowel disease (IBD) and microscopic colitis development in cancer patients receiving ICIs remains incompletely characterized. This study aimed to evaluate the association between ICI therapy and subsequent coding for de novo unspecified IBD, ulcerative colitis (UC), Crohn’s disease (CD), and microscopic colitis.
Methods:
We conducted a retrospective cohort study using the TriNetX real-world database from March 2011 to January 2025. Adult cancer patients ( >18 years) treated with ICIs targeting PD-1, PD-L1, and CTLA-4 (exposed) were compared to cancer patients not receiving ICIs (non-exposed). Patients with pre-existing IBD or microscopic colitis codes were excluded. Both groups were 1:1 propensity matched for age, gender, demographics, and cancer types. Included patients with diarrhea or colitis code within six months post-treatment. De novo IBD and microscopic colitis coding were assessed at one year post-treatment. Outcomes included unspecified IBD, any IBD (any IBD-related code), and specific codes for UC or CD.
Results:
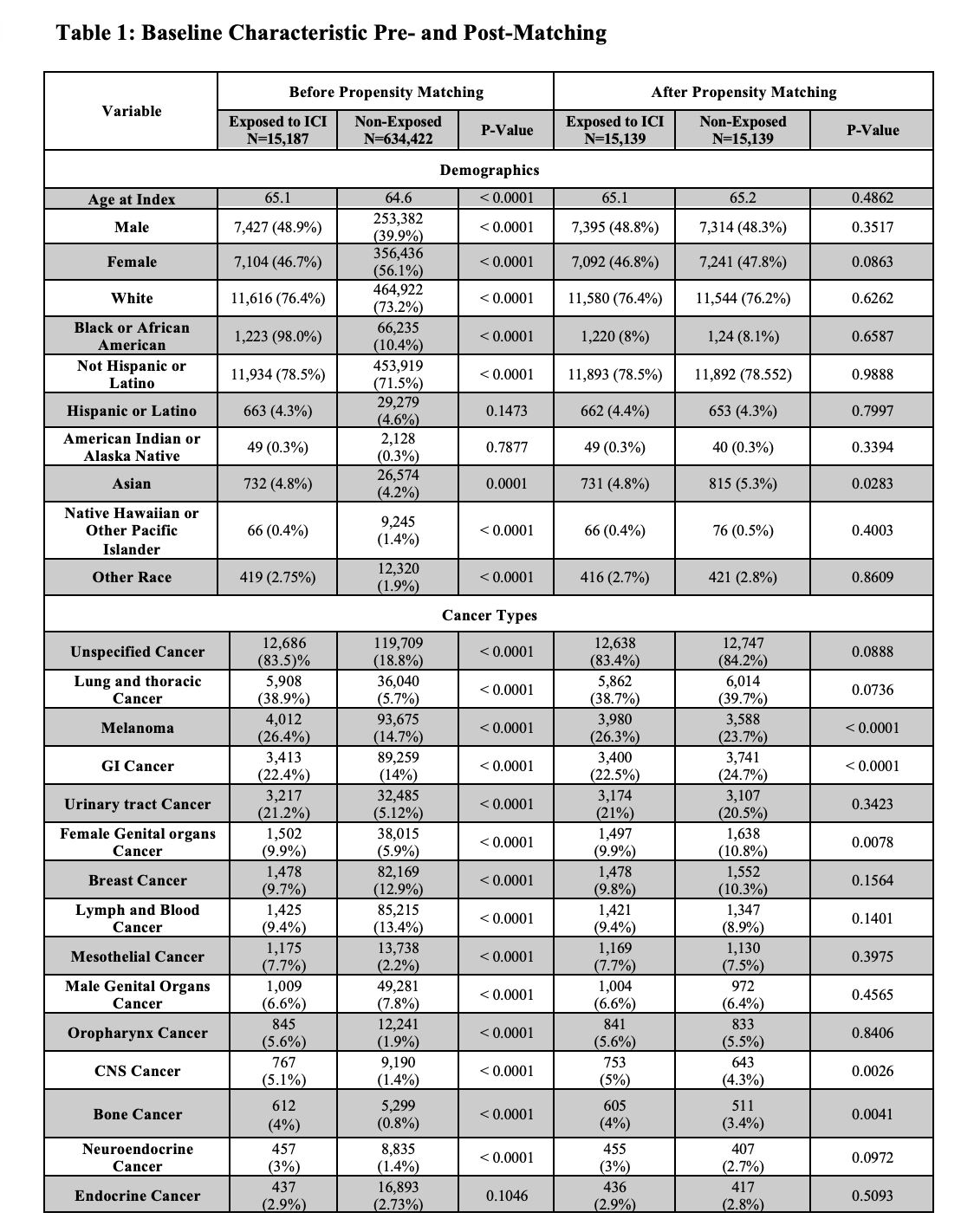
After propensity matching, both exposed and non-exposed groups included 15,139 patients each. The average age was 65 years for both groups, with females accounting for 46.8% in the exposed and 47.8% in the non-exposed (Table 1). No significant difference was observed for unspecified IBD coding (0.06% vs 0.06%, OR 1.1, 95% CI 0.4-2.4, p=1.0). Any IBD code occurred more frequently in exposed patients (9.0% vs 5.5%, OR 1.63, 95% CI 1.5-1.7, p< 0.001). ICI therapy was associated with significantly increased risk of de novo UC coding (0.6% vs 0.06%, OR 9.8, 95% CI 5.1-18.7, p< 0.001), but no significant difference in CD coding (0.11% vs 0.06%, OR 1.7, 95% CI 0.7-3.7, p=0.17). Microscopic colitis coding occurred more frequently in exposed patients (0.3% vs 0.06%, OR 5.5, 95% CI 2.8-10.7, p< 0.001).
Discussion:
Cancer patients receiving ICI therapy have a significantly increased risk of developing de novo IBD coding, with ulcerative colitis showing the highest risk (~10-fold increase). Microscopic colitis coding risk was also significantly elevated. These entities need to be considered in the differential diagnosis of ICI-related diarrhea or colitis, and future studies are needed to confirm these coding-based associations through clinical validation.
Disclosures:
Danah Al-Deiri, MD1, Anika Azem, MD1, Sara Haddad, MD2, Julia Wajsberg, MD3, Joseph Sleiman, MD1, Jessica R. Philpott, MD, PhD, FACG1. P0282 - Association Between ICI and Chronic Colon Mucosal Inflammation: IBD and Microscopic Colitis Risk, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
